Lecture #3: Resting Membrane Potential, Midwestern University Presentation
Slides from Midwestern University about Lecture #3: Resting Membrane Potential. The Pdf explains the fundamental concepts of membrane potential, equilibrium potential, and the Nernst and Goldman-Hodgkin-Katz equations, relevant for University Biology students. It also details ion channels and body fluid compartments.
Ver más10 páginas

Visualiza gratis el PDF completo
Regístrate para acceder al documento completo y transformarlo con la IA.
Vista previa
Midwestern University
@Isabel Martinez-Pena y Valenzuela Dr. I. Martinez-Pena Summer 2025
Learning Objectives
- Be able to describe the electrical and chemical forces that modulate the diffusion of an ion across cell membranes.
- Be able to explain the concepts of membrane potential and resting membrane potential. Be able to describe the general characteristics of channels and explain how channels act to regulate the movement of ions across cell membranes
- Be able to explain the concept of equilibrium potentials and how the equilibrium potentials for sodium and potassium are relevant to the resting potential and deviations of the membrane potential from the resting potential.
- Be able to explain the significance of the Nernst Equation and understand its implications for resting membrane potential.
- Be able to describe the primary determinants of the resting membrane potential and how alterations in the relevant parameters affect the resting membrane potential.
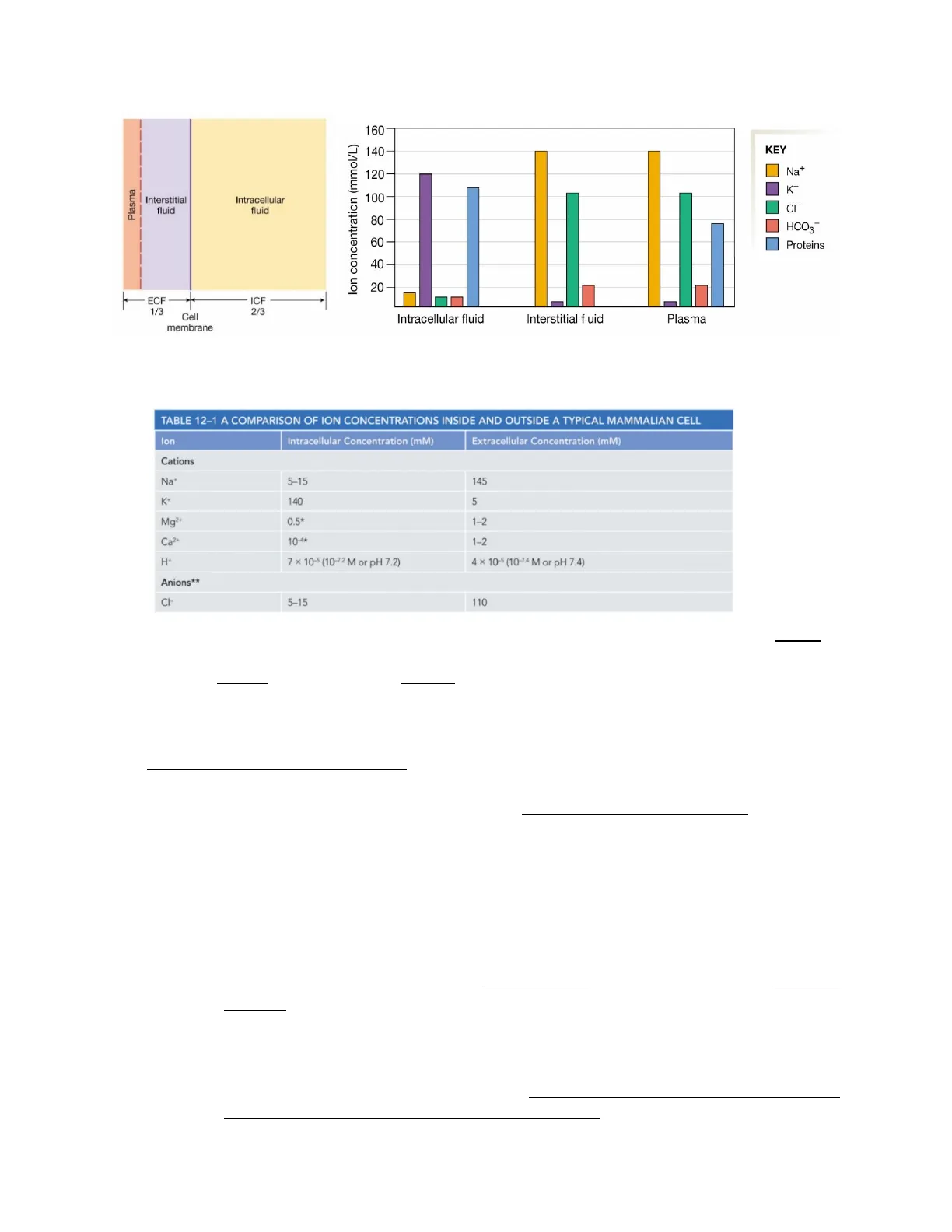
Intracellular and Extracellular Ion Concentrations
Body Fluids Compartments
The body fluids are in two compartments: the extracellular fluid (ECF) and the intracellular fluid (ICF). The ECL fluid is composed of the interstitial fluid (between the circulatory system and the cells) and blood plasma (the liquid matrix of blood). The ICF is the fluid inside cells and contributes to 2/3 of the total body water volume, the ECF is 1/3 of the total body water volume. The ECF and ICF are in osmotic equilibrium but have different chemical compositions. The cell membrane is a selectively permeable barrier between the ECF and ICF.
Lecture #3 - Resting Membrane Potential p. 3-1160
lon concentration (mmol/L) 140 Na+ 120 I K+ 100 CI" 80 HCO3 60 Proteins 40 20 Intracellular fluid Interstitial fluid Plasma
Fig. 2. The body compartments are in a state of chemical disequilibrium. The cell membrane is a selectively permeable barrier.
TABLE 12-1 A COMPARISON OF ION CONCENTRATIONS INSIDE AND OUTSIDE A TYPICAL MAMMALIAN CELL
lon Intracellular Concentration (mM) Extracellular Concentration (mM) Cations Na+ 5-15 145 K+ 140 5 Mg2+ 0.5* 1-2 Ca2+ 10-4* 1-2 H+ 7 × 10-5 (10-72 M or pH 7.2) 4 × 10-5 (10-74 M or pH 7.4) Anions ** CI- 5-15 110
- Potassium (K+) is the major cation within cells, and sodium (Na+) is the major cation in the ECF. Phosphate ions and negatively charged proteins, on the other hand, are the major anions in the ICF. Ions are not evenly distributed between the ECF and the ICF. The intracellular compartment has some more anions than the ECF giving the cells a net negative charge. At the same time, ECF has a net positive charge.
Principles of Membrane Dynamics
- Lipid bilayers are impermeable to ions, ions do not move by diffusion.
- The ion concentrations inside a cell are very different from that outside.
- Concentration gradient is the difference in concentration of a molecule between inside and outside the cell. (When the charged molecule moves down the concentration gradient, it takes net charge).
- lon movement is influenced by electrical gradients
- Ions are attracted by opposite charges
- Ions are repelled by like charges
- lon movement is a combination of concentration (chemical) gradient and electrical gradient (called electrochemical gradient).
- Differences in the concentration of inorganic ions across a cell membrane create a membrane potential.
- Both the concentration gradient and the membrane potential influence the passive transport of charged molecules. Note that these two driving forces may be acting in the same direction or opposite directions.
Lecture #3 - Resting Membrane Potential p. 3-2
Plasma Interstitial fluid Intracellular fluid - ECF 1/3 ICF Cell 2/3 membrane
Fig. 1. Body fluid compartments
KEYcell membrane + (A) (B)
Fig. 3. The distribution of ions on the opposite side of a cell membrane determines the membrane potential of that cell. (A) There is no membrane potential when the charges on either side of the membrane are exactly balanced. (B) When ions of one type cross the membrane, they establish a charge difference across the two sides of the membrane that creates a membrane potential.
Membrane Potential (Vm)
The membrane potential is the potential (voltage) difference across the cell membrane, i.e., the voltage difference between the inside of the cell and the outside of the cell. It is caused by the separation of charges across the membrane.
Creation of a Membrane Potential in an Artificial System
To show the steps in the creation of membrane potential in a cell, we use an artificial system where we can control the composition of the ECF and ICF and the membrane permeability.
1. The system is electrically neutral but in chemical disequilibrium (because there are concentration gradients for all four ions). The cell membrane is an insulator that prevents ions' free movement between compartments (Fig. 4).
1 + + 1 + 1 x x 1 - + 1 + + - + + + + KEY + Sodium ion Chloride ion
Fig. 4. The cell has no membrane potential. Na+ and Cl- ions are in the ECF, and K+ and large anions (A-) are in the ICF.
+ Potassium ion Large anion
2. Now we insert a leak channel for K+ and the cell becomes permeable to K+ only (Fig. 5). (A leak channel is a channel without a gating mechanism so ions are free to flow through the channel along the concentration gradient). K+ starts to move out of the cell down its concentration gradient. The transfer of just one K+ from the cell to the ECF creates an electrical disequilibrium. Now, the ECF has a net positive charge (+1) while the ICF has a net negative charge (-1). The cell has a membrane potential difference, with the inside of the cell negative relative to the outside.
Lecture #3 - Resting Membrane Potential p. 3-3
x- + - + 1 + 1- A leak channel is inserted 2- K+ starts leaving the cell down its concentration gradient 3- Large anions (A-) cannot follow K+ because there are no channels for them
3. Additional K+ leaves the cell, going down its concentration gradient, now the inside of the cell is more negative than the outside of the cell.
Q. How much potassium will leave the cell? A. Since potassium is an ion, we must consider its electrical gradient. Keep in mind that like charges repel and opposing charges attract.
Now the increasing negative charges inside the cell begin to attract K+ from the ECF back into the cell: this is an electrical gradient with an opposite direction from the concentration gradient.
+ + + + 4 - + - + + + - 5 - 1 - 1 + + + + 1
Fig. 6. Additional K+ leaves the cell 4- More K+ leaks 5- Now the increasing negative charges inside the cell begin to attract K+ from the ECF back into the cell
4. Electrochemical equilibrium.
+ Na+ + + + I - K+ + K+ + + + + 11 + CI- + + Efflux due to concentration gradient
Fig. 7. Electrochemical equilibrium The concentration gradient sending K+ out of the cell is exactly opposed to the electrical gradient pulling K+ into the cell (note the equal length of the arrows but in opposite direction).
The electrical gradient (negative membrane potential) that exactly opposes the concentration gradient is called the equilibrium potential for potassium (Ek), which is -90mV. If potassium was the only ion moving, the potential would stabilize at -90mv (at the equilibrium potential for potassium, Ex). However, positively charged sodium ions leak into the neuron, which slightly offsets the negative charge and raises the voltmeter reading to -70 mV.
Lecture #3 - Resting Membrane Potential p. 3-4
+ + 2 + - - 1 + + 3 + + + + Fig. 5. Leak channel for K+
O + Influx due to electrical gradient
+In summary, we can see the generation of a potential difference across a membrane that contains only potassium channels in Figure 8. This is a first approximation to what happens in most cells at rest, leading to a resting membrane potential.
Fig. 8. Generation of a potential difference. Arrows represent ion movements. (b) Note there is no channel for sodium.
(c) (a) Compartment 1 Compartment 2 0.15 M 0.15 M NaCI KCI + I- K+ Na+ K+ K+ Na+ d) K K+ Na+ (e) K + K + Na+
Membrane Potential Measurement
To measure the difference in ions between the ECF and the ICF, we can place electrodes in the cell and the ECF (Fig. 9).
Fig. 9. Membrane potential measurement.
+ + + + + - + + 1 - + + 1 + + - Intracellular fluid Extracellular fluid Absolute charge scale + -2 -1 0 +1 +2
Fig. 10. Membrane potential measurement.
- + + + 1 + + - + + + + + - - Intracellular fluid Extracellular fluid Absolute charge scale -- -2 -1 0 +1 +2 Intracellular fluid Extracellular fluid Relative charge scale 1 extracellular fluid set to 0. -2 -1 0 +1 +2
The membrane potential is normally measured with electrodes (Fig. 11). Units of measurement are generally mV.
Lecture #3 - Resting Membrane Potential p. 3-5
On a number line, the ECF would be at +1 and the ICF at -1 BUT in real life, we measure the difference between the two electrodes, and by convention, the EFC is considered the ground and always set at 0 mV. This gives the ICF a relative charge of -2 (Fig. 10).
The Resting Membrane Potential (Vm)
This is the membrane potential "at rest". That is when the cell is not firing action potentials. Inside negative compared to outside. Most cells in the human body have resting membrane potentials around -70 mV because the cell is permeable primarily to K+. Positive Vm values can be observed in some excitable cells at the peak of an action potential.
The Na-K-ATPase helps maintain the resting membrane potential by removing Na+ that leaks into the cell and returning K+ that has leaked out (Fig. 12). Note that this means that this pump is electrogenic (i.e., the pumping itself moves some charge and, thus, contributes a very small amount to the resting membrane potential; this small amount, typically only a few mV, is usually ignored).
The voltmeter measures the difference in electrical charge between the inside of the cell and the bath solution. This value is the membrane potential difference (Vm) A recording electrode is placed inside the cell Input -70 0 The ground or reference electrode is placed in a bath Output The membrane potential can change over time +40 Membrane potential difference (V.) +20 Membrane potential (mV) 0 -20 40 Vm If the membrane potential becomes less negative than the resting potential, the cell depolarizes -60 -80 Depolarization -100 Hyperpolarization -120 Time (msec) ->
Fig. 11. Measuring the membrane potential. In the laboratory, a cell's membrane potential is measured by placing one electrode inside the cell and a second in the extracellular bath. The ground or reference electrode is given a value of 0 millivolts (mV).
Na+ Na+ Intracellular fluid -70 mV ATP K+ K+ Extracellular fluid 0 mV
Fig. 12. Most cells in the human body are about 40 times more permeable to K+ than to Na+, and the resting membrane potential is about -70 mV. Na-K-ATPase helps maintain the resting membrane potential
Significance of Membrane Potential Changes
Because the membrane potential changes are the electrical signals the body uses for communication.
Equilibrium Potential Definition
As explained above, it is the voltage at which the flux of a particular ion due to the concentration gradient for the ion balances the opposing flux due to the electrical gradient for that ion. Also known as the reversal potential.
A potential differential (electrical gradient) is produced while an ion moves. The flux caused by the electrical gradient setup, which prevents the ion from moving further, eventually
Lecture #3 - Resting Membrane Potential p. 3-6
If the membrane potential becomes more negative, the cell hyperpolarizes Repolarization