Enzymes: Structure, Induced Fit Model, and Activity Factors
Slides from University about Enzymes. The Pdf explores the fundamental aspects of enzymes, defining them as protein molecules with tertiary and quaternary structures. It also details the induced fit model of Koshland and examines key factors like temperature and pH that influence enzyme activity, relevant for University Biology students.
See more28 Pages
Unlock the full PDF for free
Sign up to get full access to the document and start transforming it with AI.
Preview
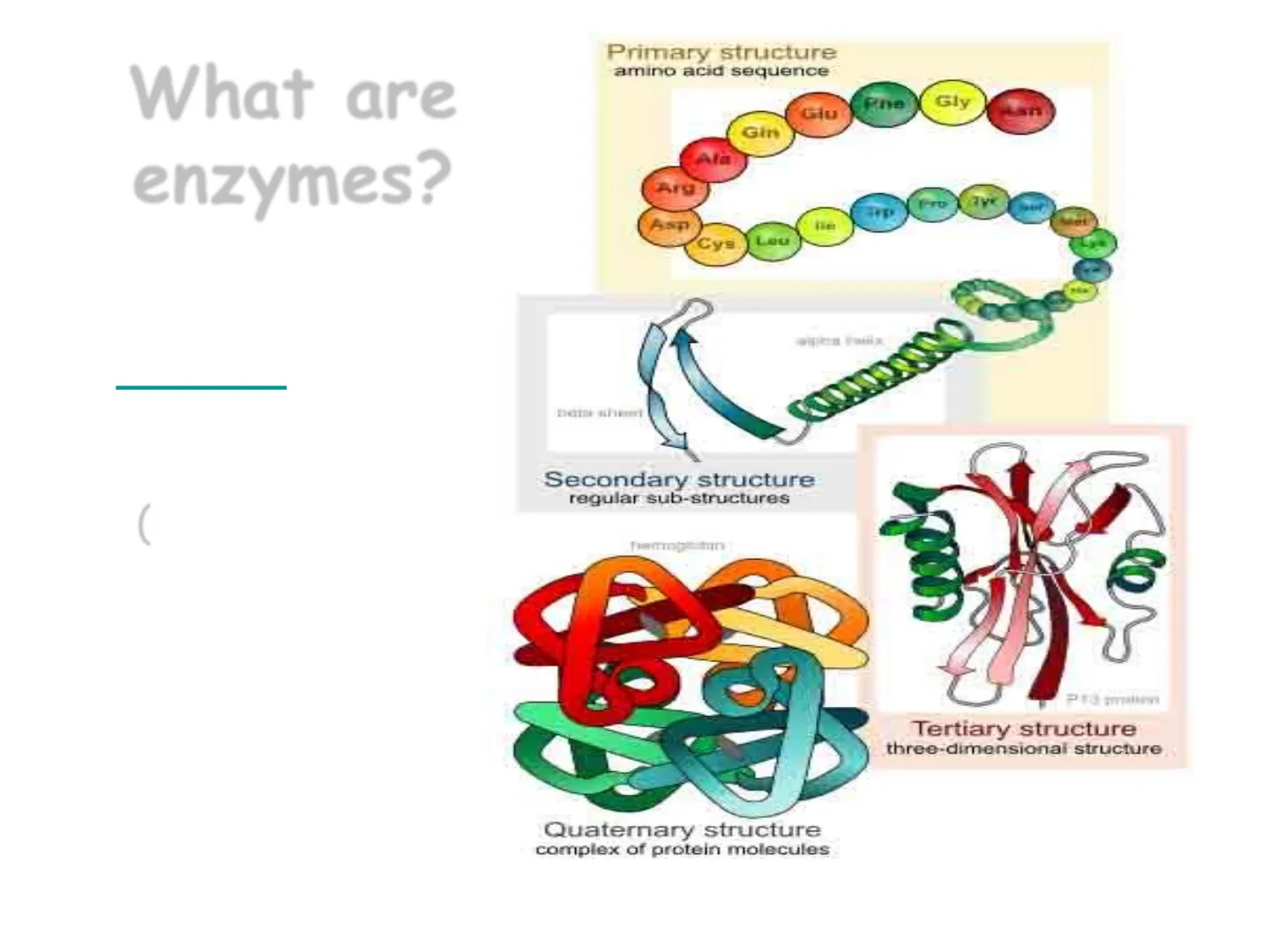
What are enzymes?
Enzymes are Protein molecules (tertiary and quaternary structures).
Primary structure
amino acid sequence Gly Giu Gin Afa Arg Pro Cys. Lou
Secondary structure
regular sub-structures
Tertiary structure
three-dimensional structure|
Quaternary structure
complex of protein molecules
What do enzymes do?
- Enzymes act as catalysts in cellular reactions.
without enzyme activation energy without enzyme with enzyme activation energy with enzyme Energy reactants e.g. C 6H1206 + O2 overall energy released during reaction products CO2+H2O
Q: What does a catalyst do? Reaction coordinate Catalysts are chemicals that take part in facilitating reactions by reducing the energy of activation.
How do enzymes work?
- Reaction without catalyst
- Reaction with catalyst
Ea (-)YX Ea (-)XY Energy Y X Reaction path Enzymes catalyze reactions by weakening chemical bonds, which reduces activation energy.
Catalysts
A Sem catalisador BI Com enzimas Com catalisador inorgânico Energia Sentido da reacção Activation energy required for chemical reactions: without the action of a catalyst --; with the action of an inorganic catalyst -- and with the action of enzymes --
Enzymes are very specific about which reactions they catalyze. Only molecules with exactly the right shape will bind to the enzyme and react. These are the substrate molecules. The part of the enzyme to which the substrate binds is called the active site. This is a very specific shape and the most important part of the enzyme. Substrate Active site JC Revy / SPL
How do enzymes work?
- Each enzyme has a unique 3-D shape, including a surface groove called an ACTIVE SITE.
- The enzyme works by binding a specific chemical reactant Enzime Reactant Complex to its active site, causing the substrate to become unstable and react.
- The resulting products(s) is then released from the active site. (a) Reaction Substrate Active site Enzyme Enzyme binds substrate Enzyme releases products From the Virtual Cell Biology Classroom on ScienceProfOnline.com Image: Enzymatic reaction, Jerry Crimson Manni
Enzyme Cofactors
Some enzymes require cofactors to be active. Cofactors are a nonprotein component of an enzyme. Cofactors can be: organic molecules (coenzymes). inorganic ions (e.g. Ca2+, Zn2+). Cofactors may be: Permanently attached, in which case they are called prosthetic groups. Temporarily attached coenzymes, which detach after a reaction, and may participate with another enzyme in other reactions. Active site Enzyme is protein only Example: lysozyme Enzyme Active site Prostheti c group Enzyme Enzyme + prosthetic group Example: flavoprotein + FAD Active site Coenzym e Enzyme Enzyme + coenzyme Example: dehydrogenases + NAD
Enzyme-Substrate Linkage Models
Lock and Key Model
Substrate Active Site Products Enzyme Enzyme-Substrate Complex Enzyme-Products Complex Enzyme By Emil Fischer in 1894. The shape of the active center is perfectly complementary to that of the substrate, which fits like a key in a lock. It does not explain the relative specificity.
Induced Fit Model
Substrate Active Site Enzyme adjust its shape Products Enzyme Enzyme-Substrate Complex Enzyme-Products Complex Enzyme by Daniel E. Koshland, Jr., in 1958. The shape of the active center adapts to fit the substrate. Dynamic model. Explains relative specificity and admits a more perfect fit of substrate to enzyme.
Factors That Influence Enzyme Activity
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- Presence of inhibitors Image: Animation of Enzyme, Wiki
Temperature
- Each enzyme has optimum operating temperatures. As the values move away from the optimum, activity decreases. Low temperature:
- Reversibly inhibits the enzyme.
- Increases the rigidity of the active center and hinders binding to the substrate. High temperature
- Irreversible inhibition.
- Destruction of intermolecular bonds that maintain the three-dimensional structure - denaturation of the enzyme increasing enzyme activity optimum temperature 0 10 20 30 40 50 60 70 temperature (º℃)
Temperature vs Enzyme Activity
Increasing rate of Reaction Optimum temperature Increasing enzyme activity Enzyme being denatured 10 20 30 40 50 Temperature (C)
pH
Each enzyme has optimal values depending on the medium to which they are adapted. pH - changes the distribution of the electrical charges of the enzyme, and can modify the bonds that maintain the three- dimensional structure. When they are extreme values it may induce irreversible denaturation. Optimum pH Increasing enzyme activity 4 5 6 7 8 9 10 11 PHEffect of pH on Enzymes 100 Enzyme Activity (%) 60 Pepsin Catalase Lipase 40 20 0 0 1 4 5 8 7 9 10 11 12 PH
Enzyme and substrate concentration
Enzyme concentration The higher the concentration, the more enzyme is available to catalyze the reaction. Substrate concentration Depends on enzyme concentration. The speed increases until all the enzymes are occupied. When this happens the speed stabilizes - the saturation point is reached. reaction speed Velocidade da reacção ~> C > Enzyme concentration da reacção ~> Velocidade D Substracte tration .. ]
Enzyme inhibition
An inhibitor is any substance that slows down or stops an enzyme reaction, affecting the enzyme in some way. IRREVERSIBLE or NON-REVERSIBLE REVERSIBLE The enzyme becomes totally inactive. The cells have to produce more enzymes of the same type. E.g .: Heavy metals like Pb or Hg form covalent bonds with the S of some proteins, becoming poisons. Substances that temporarily bind to enzymes, and whose inhibitory effect can be reversed. There are two groups of reversible inhibitors.
- Competitive inhibitors
- Non-competitive inhibitors
Enzyme inhibition Competitive
- The inhibitor competes with the substrate for the active center of the enzyme.
- By increasing the substrate concentration, the inhibition is reduced. (a) Reaction Substrate Active site Enzyme - Enzyme binds substrate Enzyme releases products (b) Inhibition Inhibitor Active site Enzyme Enzyme binds inhibitor Inhibitor competes with substrate
Enzyme inhibition Competitive Example
Antabuse EXAMPLE: The drug Antabuse is used to help alcoholics quit drinking. Antabuse inhibits aldehyde oxidase, resulting in the accumulation of acetaldehyde during the metabolism of alcohol. Elevated acetaldehyde levels cause symptoms of nausea and vomiting.
Enzyme inhibition Non-competitive
(a) Reaction substrate active site enzyme (b) Inhibition Substrate molecule binds to active site of an enzyme molecule Reaction occurs and product molecules are generated substrate active site enzyme inhibitor Inhibitor molecule binds to a part of enzyme other than active site Than Inhibitor prevents the binding of substrate by changing the shape of active site The inhibitor binds to a site other than the active center - the allosteric center. Binding to the allosteric center changes the conformation of the enzyme's active center.
Substrato Inibidor Enzima
Substrato 1 S I S Competitive inhibition Normal enzyme Rate of reaction Competitive inhibitor Noncompetitive inhibitor Substrate concentration The presence of the inhibitor does not stop the enzyme activity. The enzyme always has the active center occupied, by substrate or inhibitor.
Substrato Inibidor Enzima Allosteric
Substrato S I S Alteração do centro activo Allosteric inhibition Normal enzyme Rate of reaction Competitive inhibitor Noncompetitive inhibitor Substrate concentration The presence of the inhibitor stops the enzyme activity.
Inhibitor drugs
Medicinal drug Inhibitory action Benefit of the inhibitor Penicillin Irreversible inhibition of the enzyme trans-peptidase, which is a player in the bacterial cell wall Death of bacteria Aspirin Irreversible inhibition of the enzyme COX, involved in the production of prosta-glandins to stimulate pain and inflammation Reduces inflammation and relieves pain Eflornithine Non-reversible inhibitor of ornithine decarboxylase, a key enzyme in cell growth Used in the treatment of sleeping sickness (African trypanosomiasis) Statins Competitive inhibitor of HMG-CoA reductase, an enzyme involved in cholesterol synthesis, in the liver Reduction of the cholestorol concentration in the blood
Anabolic enzymes
catalyze biochemical reactions that synthesize larger complex molecules from smaller units.
- Photosynthesis
- Protein biosynthesis. Require energy and consume ATP - Endergonic i.e.anabolic enzymes include DNA polymerase, RNA polymerase, ATP synthase
Catabolic enzymes
catalyze biochemical reactions that break down larger complex molecules into smaller units.
- Digestion
- Respiration Reactions that are catalyzed by catabolic enzymes release energy and generate ATP - Exergonic i.e. catabolic enzymes: lipase, sucrase, lactase, and protease.
Metabolic pathway control
A e1 B e2 C e3 e4 D E A metabolic pathway consists of a series of chain reactions controlled by enzymes. A e1 A e2 + B e3 inhibition C . D - e4 E (End product) Metabolic pathways are often controlled by the end product. This process occurs through negative feedback. What will happen if the concentration of product E decreases? What type of inhibition is involved in a metabolic pathway?
Inhibition of the pathway
Substrate Intermediate substrate A Intermediate substrate B End product Enzyme 1 Enzyme 2 Enzyme 3 Feedback inhibition: Metabolic pathways are a series of reactions catalyzed by multiple enzymes. Feedback inhibition, where the end product of the pathway inhibits an earlier step, is an important regulatory mechanism in cells.
Enzyme Classification
Usually, an enzyme is named using the name of the substrate followed by the suffix "ase". E.g .: Protease, amylase, lipase. There are, however, some enzymes whose names do not follow this rule. E.g. trypsin, pepsin. Enzymes are classified according to the type of reaction they catalyze. There are 6 major groups.
- Hydrolases - catalyze the hydrolysis of substrates by adding a water molecule. They include the digestive enzymes that act in the human digestive tract.
- Oxyreductases - participate in oxidation-reduction reactions.
- Transferases - transfer a group of atoms from one molecule to another.
- Isomerases - control the conversion of one isomer into another by transferring a group of atoms from one molecule to another.
- Liases - break chemical bonds without the addition of water (hydrolysis).
- Ligases - catalyze reactions in which new chemical bonds are made, using ATP.