Transport Across the Plasma Membrane: Diffusion, Osmosis, and Active Transport
Slides about Transport Across the Plasma Membrane. The Pdf, a university-level Biology document, details cell permeability, diffusion, osmosis, and osmotic pressure, using diagrams to illustrate molecular movement across the membrane.
See more48 Pages
Unlock the full PDF for free
Sign up to get full access to the document and start transforming it with AI.
Preview
Transport Across the Plasma Membrane
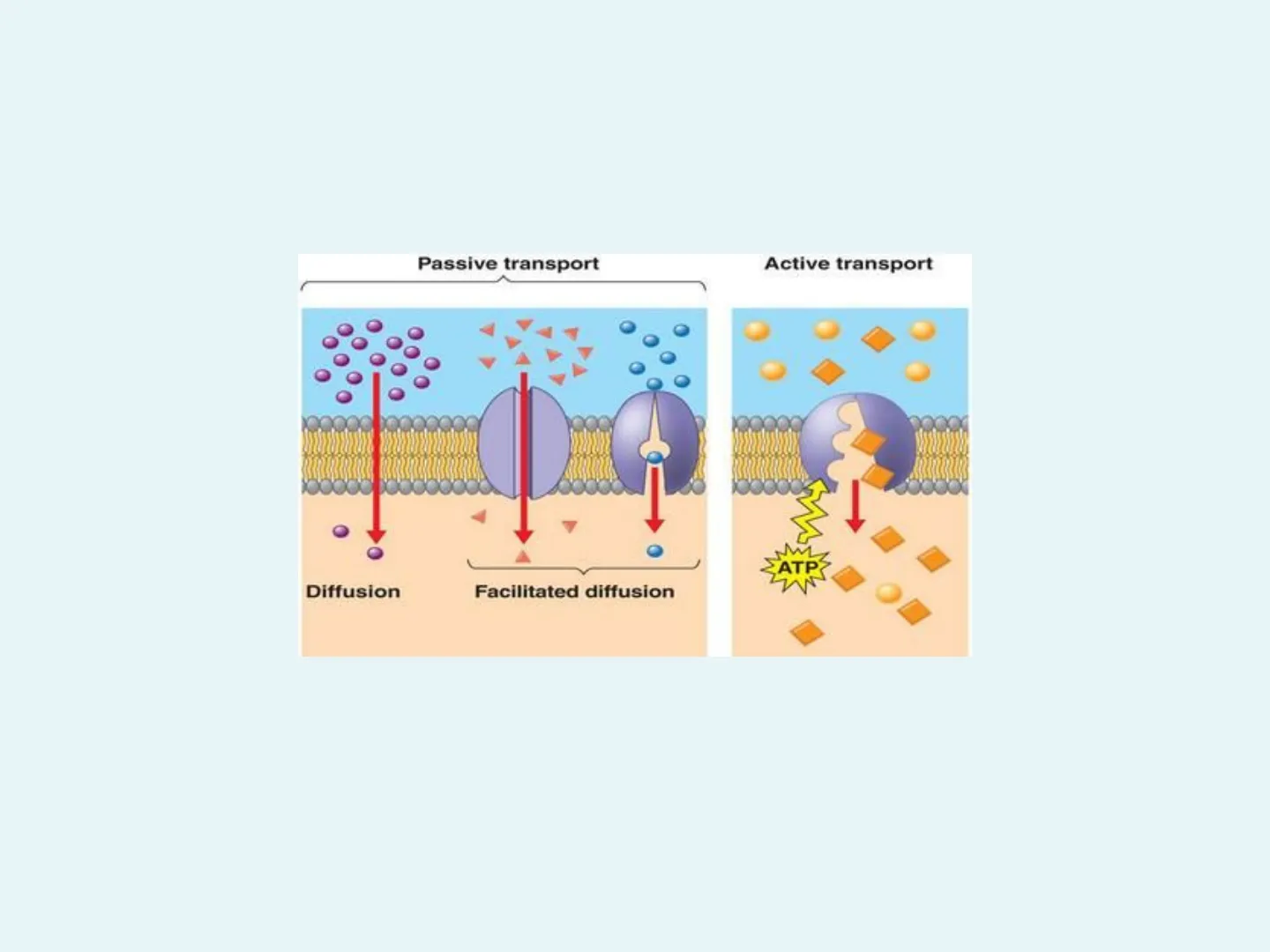
Transport Across the Plasma Membrane Passive transport Active transport ATP Diffusion Facilitated diffusion Diffusion, Osmosis, Facilitated Transport, Active Transport, Exocytosis, EndocytosisCell Permeability Permeability refers to whether or not a membrane will allow molecules across.
- Permeable - the membrane allows a substance to cross through.
- Impermeable - the membrane does NOT allow a substance across.
Cell membranes are Differentially (Selectively) Permeable in that they will allow some substances to cross, whereas other substances will not be allowed through.
Diffusion
Movement of Solute Particles
2Diffusion 3Diffusion . The movement of solute particles from an area of higher solute concentration to an area of lower solute concentration.
- Molecules diffuse "down" or "with" their concentration gradients.
- Diffusion stops once a dynamic equilibrium is reached.
4· Diffusion is a passive process (no energy is required). Examples:
- Small lipid-soluble molecules
- oxygen and carbon dioxide gases
- water
5The molecules spread out until they are evenly distributed
61 Lump of sugar 2 3 Sugar molecule A lump of sugar is dropped into a beaker of water. Sugar molecules begin to break off from the lump. More and more sugar molecules move away and randomly bounce around. 4 Eventually, all of the sugar molecules become evenly distributed throughout the water.
7WHAT IS DIFFUSION?
Factors Affecting Diffusion Rate
Size and Shape of Solute Molecules
8Factors that affect the RATE of Diffusion
91. Size and Shape of the solute molecules Smaller molecules diffuse quicker!
Solubility of Solute Molecules
102. Solubility of the solute molecules in the solvent . This means how easily the solute (ex. sugar) will dissolve into the solvent (ex. water). · When a substance readily dissolves in the environment (either inside or outside of the cell), it will diffuse faster. · greater solubility = faster diffusion Solute Solvent Solution
Temperature and Diffusion
113. Temperature hotter temperature = faster diffusion 500 PYRE
Permeability of Cell Membrane
124. Permeability of the cell membrane to the solute molecules . The more permeable the cell membrane the faster the rate of diffusion.
Concentration Gradient
135. Concentration Gradient between two regions . The greater the concentration gradient = the faster the diffusion of solute molecules down the gradient · A greater concentration gradient means that the solute concentration on either side of the membrane is very different (high on one side and low on the other side)
14Concentration Gradient . The difference in concentration of molecules across a space Think of concentration gradient like a hill: The steeper the hill, the faster you move down it on your skateboard! Low High Concentration Concentration
Osmosis
Water Movement
15Osmosis
16· Osmosis is the diffusion of WATER. · Water moves from a higher "water concentration" toward a lower "water concentration". · A higher "water" concentration means a lower concentration of dissolved solutes. . Therefore, water moves TOWARD the area with the higher "solute" concentration!
17Concentrated sugar solution (Water less concentrated) O 0 O O O O O O O 0 O o O O O O Selectively permeable membrane Dilute sugar solution (Water more concentrated) O O O O Sugar molecules O Movement of water
Osmotic Pressure
18 OOsmotic Pressure is the force that causes water to move into an area. The higher the solute concentration of an area, the higher the osmotic pressure that is exerted, and the greater the tendency for water to move into that area by osmosis. The membrane allows water molecules to diffuse through Water molecules The solute cannot pass through the membrane Water molecules are attracted to the solute Osmosis across a cell membrane
Tonicity of Solutions
Isotonic Solutions
19Tonicity of Solutions
20Isotonic Solutions · have the SAME solute concentration. · A cell placed in an isotonic solution will gain and lose the same amount of water, so there will be NO NET change. Solution is Isotonic . 10% Salt Water goes in both directions 10 % Salt . Water Molecules Isotonic Amount of water transported into the cell equal to the amount of water transported out from the cell O H2O 2 H2O O Solute concentration inside the cell is Equal to the solution outside the cell Flaccid
Hypertonic Solution
21Hypertonic Solution · Has a GREATER solute concentration. · A cell placed in a hypertonic solution will LOSE water to the more concentrated solution. . The shrunken cell is said to be crenated in an animal cell and plasmolyzed in a plant cell. Solution is Hypertonic 20% Salt Water moves out of the cell 10 % Salt . Water Molecules Hypertonic Water is transported out from the cell Solute concentration inside the cell is LOWER Plasmolyzed
Hypotonic Solutions
22Hypotonic Solutions · Have a LOWER solute concentration. · A cell placed in a hypotonic solution will GAIN water because the cell has a higher solute concentration and exerts a greater osmotic pressure than the solution. . The cell will swell, plant cells become turgid, and animal cells may burst (lysis). Solution is Hypotonic 10 % Salt Water moves into the cell 20% Salt Water Molecules Hypotonic Vacuole Water is transported into the cell HO H,O 2 O Solute concentration inside the cell is HIGHER Turgid
Tonicity Comparison
23Remember Tonicity words are comparison words and can't be used alone. - If the cell is hypertonic, then the solution that surrounds the cell must be hypotonic. - If the cell is hypotonic, then the solution that surrounds the cell must be hypertonic. and so on ...
24https://llunadeneu.wordpress.com/2017/10/03/water-and-mineral-salts/ Isotonic solution (normal) Hypotonic solution (dilute) Hypertonic solution (concentrated) O C 0 O O C O O O O Solute C C O O o 0 A Normal red blood cell B Swollen red blood cell C Shrunken (crenated) red blood cell - Direction of osmotic water movement
25 WaterSemipermeable MeMbraNe Osmosis With the Amoeba Sisters
26ATT N
Facilitated & Active Transport
Facilitated Transport Overview
27Facilitated & Active Transport
28Facilitated Transport · Also called facilitated diffusion or passive transport. · Requires specific channel proteins to assist with the passage of molecules across the cell membrane. · Molecules move from high to low concentration, as in diffusion.
Facilitated Diffusion Process
29NO ENERGY is required since the molecules move with the concentration gradient. Facilitated Diffusion Outside of cell x Xx Inside of cell . Once the solute molecule has passed through, the carrier protein may assist more solute molecules through the cell membrane. · These carrier proteins are reversible
Examples of Facilitated Diffusion
30Examples: .Glucose ·Amino acids These molecules are not lipid soluble so can't pass through the cell membrane without a carrier protein.
31Facilitated diffusion Outside cell Outside cell Low concentration of solute High concentration of solute Inside cell Inside cell
Active Transport Overview
32Active Transport · Requires specific carrier proteins to move ions or molecules through the cell membrane. · Used to accumulate solute either on the inside or outside of the cell. · Molecules move against the concentration gradient.
Energy for Active Transport
33· ENERGY is required to move molecules from regions of low to high concentrations. · The energy helps the carrier protein to "change" its shape. · Energy is provided by molecules of ATP (Adenosine Triphosphate). · Cells involved in a lot of active transport have many mitochondria (which produce ATP). . lodine, Na+ ions and K+ ions all move by active transport + EXTRACELLULAR FLUID high + ATP H* concentration Proton pump H+ concentration H+ - + H+ - + H+ CYTOPLASM pH is higher [H+] is lower + pH is low [H+] is higher @1999 Addison Wesley Longman, Inc. H+ lowPassive transport Active transport Diffusion Facilitated diffusion ATP
35Diffusion Passive transport Facilitated diffusion - ATP Active transport
Sodium-Potassium Pump Mechanism
36Sodium-Potassium Pump 1 Na+ Na P Na+ P P ATP The sodium-potassium pump binds three sodium ions and a molecule of ATP. 2 K K Na Na Na P P P A ADP The splitting of ATP provides energy to change the shape of the channel. The sodium ions are driven through the channel. 3 Na Na Na K K P P P A ADP The sodium ions are released to the outside of the membrane, and the new shape of the channel allows two potassium ions to bind. 4 P K A K Release of the phosphate allows the channel to revert to its original form, releasing the potassium ions on the inside of the membrane.
Endocytosis & Exocytosis
Exocytosis Process
37Endocytosis & Exocytosis
3839Plasma membrane Extracellular fluid Cytoplasm Material for secretion Secretory vesicle (from Golgi apparatus) Molecules are expelled out of the cell when a vesicle fuses with the cell membrane. The vesicle "breaks open" and releases the molecules to the outside of the cell. EXOCYTOSIS The vesicle becomes part of the cell membrane when it fuses, so exocytosis is part of normal cell membrane growth.
Exocytosis Examples
40Exocytosis · Hormones, neurotransmitters and digestive enzymes are secreted from a cell in this way. . The Golgi apparatus often produces these secretory vesicles that carry the molecules to the cell membrane.
Endocytosis Process
41Endocytosis . Endocytosis is the intake of large particles or small molecules into a cell by an infolding of the cell membrane. . After the cell membrane "wraps" around the particle, it fuses and pinches off a vesicle (or vacuole), which contains the particle inside.
42ENDOCYTOSIS OUTSIDE THE CELL 2 Cell membrane 1 3 CYTOPLASM A Lysosome then fuses with the vesicle and digests the vesicles' contents, which then allows the molecules to enter the cell cytoplasm. Vesicle
Types of Endocytosis
43Types of Endocytosis Phagocytosis · Taking in large particles or small cells. · Occurs in certain white blood cells in humans. · Common in unicellular organisms like amoebas. · Used by Immune cells in people. Pinocytosis · Taking in of extracellular fluids and the small particles within the fluid. . These solutes have already been dissolved in the fluid. · non-specific process. . Occurs continuously in all cells.
Receptor-mediated Endocytosis
44Receptor-mediated Endocytosis Receptor-mediated endocytosis Coat protein Receptor Coated vesicle 1 Coated pit ·Specific molecule
45Certain molecules, like hormones, vitamins and lipoproteins, enter a cell by pinocytosis which involves specific receptors on the cell membrane. The receptors for the molecule are found at certain locations on the cell membrane of the cell called coated pits. This location is called a coated pit because the inner surface of the cell membrane has a special coating of proteins, so the vesicle that is formed is called a coated vesicle. Once the molecule is taken into the vesicle, the coating is removed so that a lysosome may fuse with the vesicle. Used during the transfer of many molecules between the maternal circulation and the fetal blood.