Unit 13: Rates & Equilibrium in Chemistry
Document from High school about Unit 13: Rates & Equilibrium. The Pdf provides a comprehensive overview of chemical kinetics and equilibrium, covering key definitions, exothermic and endothermic reactions, and the calculation of equilibrium constants. It is suitable for high school Chemistry students.
Mostra di più30 pagine
Visualizza gratis il Pdf completo
Registrati per accedere all’intero documento e trasformarlo con l’AI.
Anteprima
VOCABULARY
product
a substance formed in a chemical reaction
reactant
a substance present at the start of a reaction
activation energy (Ea)
the minimum energy colliding particles must have in order to react
catalyst
a substance that increases the rate of reaction by lowering the activation
energy without being used up in the reaction
Δ
heat
indicates that heat is supplied to the reaction
Pt
a formula written above or below the yield sign indicates its
use as a catalyst (in this example, platinum)
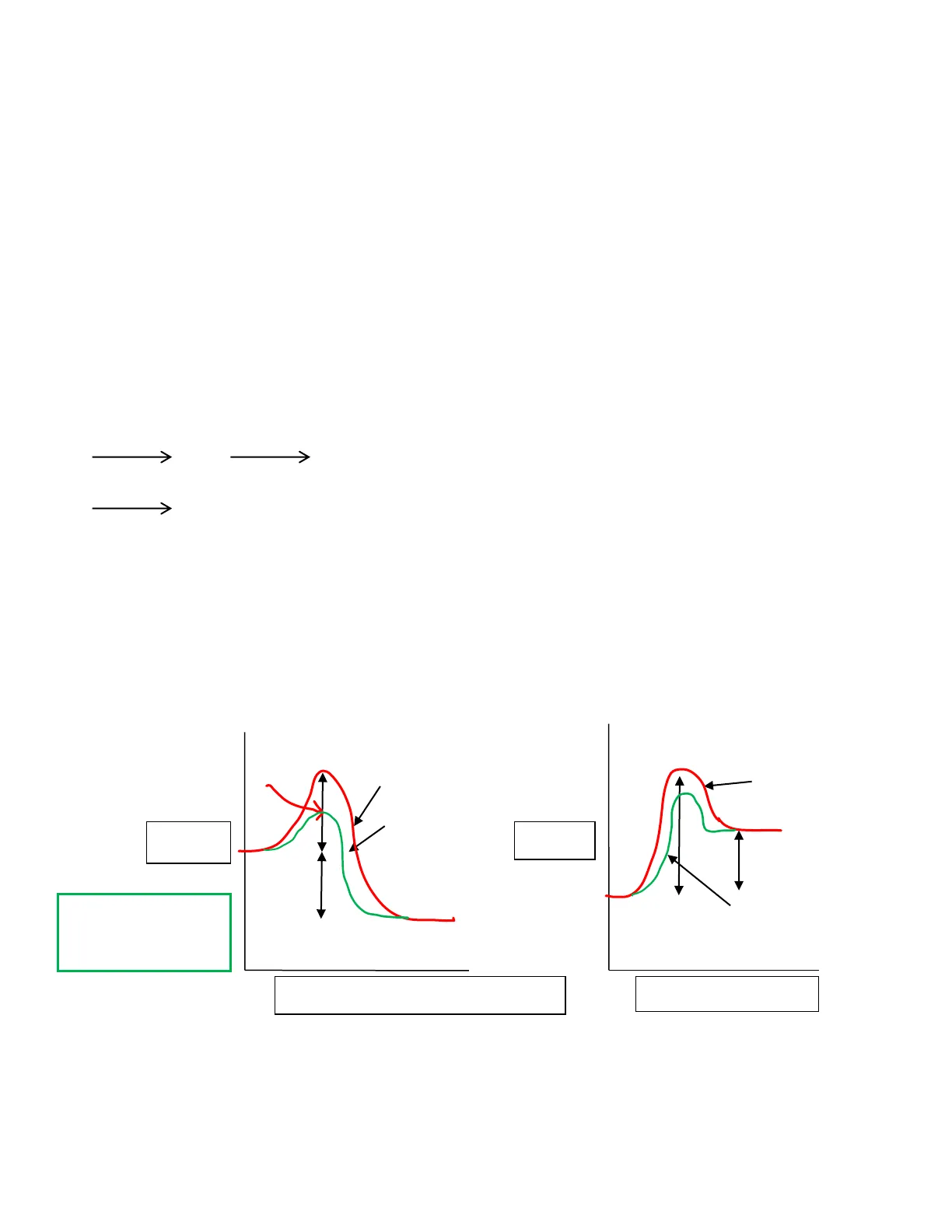
ENERGY OF CHEMICAL REACTIONS
R = reactants, P = products
exothermic reaction: AH is negative (-)
(heat is a "product") reaction feels hot
4 = change. H = heat or enthalpy
endothermic reaction: AH is positive (+)
(heat is a "reactant") reaction feels cold
activated complex (AC)
uncatalysed
AC
Ea
uncatalysed
-P
energy
Ea
ΔΗ
R
catalysed
AH = P-R
Ea = AC - R
P
reaction coordinate (= progress)
reaction coordinate
Hons Chemistry
1
catalysed
energy
R
ΔΗ
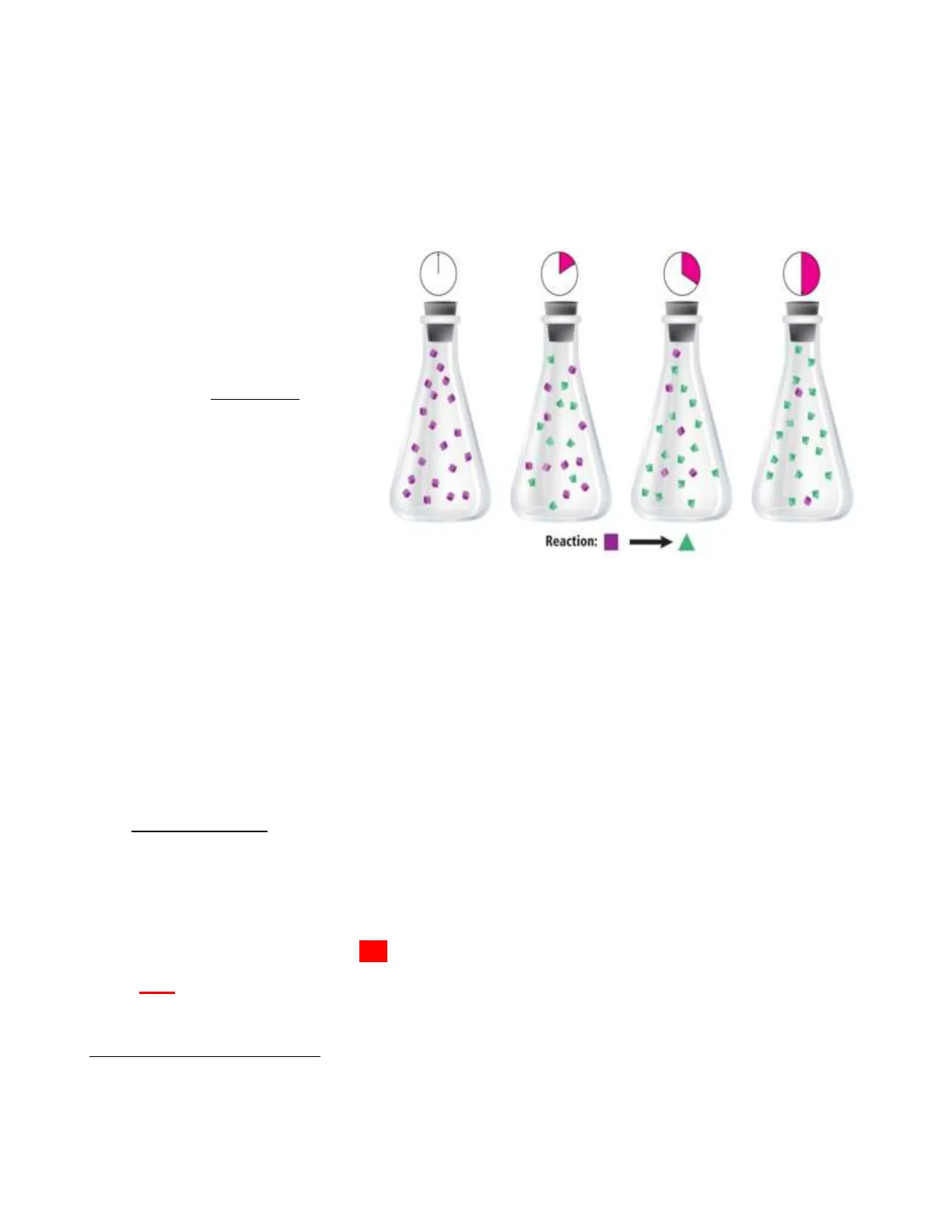
RATE OF CHEMICAL REACTIONS
The rate of a chemical reaction is the change in concentration of a reactant or product per unit of
time.
Reaction rates are determined
experimentally:
average rate = - A [reactant]
At
(Change in amount of a product
is without the negative sign)
Reaction:
The rate of a reaction increases when the # of collisions increases.
Collision Theory
- Particles must collide in order to react.
- Particles must have sufficient kinetic energy (activation energy) to react. Otherwise they
will just bounce off. - Particles must have the correct orientation (positioning)
(An "activated complex" is an unstable arrangement of atoms that forms momentarily (typically about
10-13 seconds) at the peak of the activation-energy barrier in which old bonds are breaking and new
bonds are forming.
High activation energy means that
rate is slow.
few / many collisions have the required energy and the reaction
Spontaneity and Reaction Rate
Gibbs free energy, AG, indicates only the natural tendency for a reaction to proceed - it does not affect
the rate of a chemical reaction.
Hons Chemistry
2
Factors Affecting Collision Rate
What factors may cause the # of collisions to increase?
- The nature (identity) of reactants
Some substances react more readily than others. For example, lithium reacts faster with
acids than copper because it is higher on the activity series. So the identity of a reactant is
important. - Temperature
Raising the temperature gives the reactants more kinetic energy making them move faster,
resulting in more collisions, so rate increases. Both force & frequency of collisions
increases. Decreasing the temperature slows the reaction. - Concentration of the reactants
Is the amount of solute dissolved in the solvent.
1) As more solute is added (to a constant volume of solution) concentration increases so
there are more frequent collisions, so rate increases.
2) For solutions, heating evaporates some solvent so volume decreases and concentration
increases, so more frequent collisions. - Particle size (surface area) of the reactants
Breaking or crushing a solid reactant gives a greater surface area and more frequent
collisions, so rate increases. - Adding a catalyst or inhibitor to the reaction
A catalyst lowers the activation energy so rate increases. More molecules have sufficient Ea,
so frequency of successful collisions increases.
An inhibitor raises the activation energy so frequency of successful collisions decreases, or
it interferes with the action of a catalyst.
A heterogeneous catalyst exists in a physical state different than that of the reaction it catalyzes.
A homogeneous catalyst exists in the same physical state as the reaction it catalyzes.
http://www.youtube.com/watch?v=IkqoBbFZV4Q
orientation
effective collisions
http://www.youtube.com/watch?v=OkGzaSOkyf4
Hons Chemistry
3
REACTION RATE LAWS
A rate law expresses the relationship between the rate of a chemical reaction and the concentration of
the reactants.
One-Step Reaction Rate Law
rate = k [A]
k is a constant, [A] is the concentration of a reactant
The rate of a one-step reaction is the product of a constant and the concentration of a reactant.
The symbol k is the specific rate constant, and is unique for every reaction a numerical value that relates
the reaction rate and the concentrations of reactants at a given temperature.
The reaction order for a reactant defines how the rate is affected by the concentration of that reactant.
For example, consider:
2 H2O2 > 2 H2O + O2
[H2O2] v. Initial
Reaction Rate
Rate = k [H2O2]
The reaction is first order,
so the rate changes in the
same proportion the
concentration of H2O2 changes.
[H2O2] (mol/L)
3.00
2.00
1.00
0
0.200 0.400 0.600 0.800
Initial reaction rate x 105
(mol/L·S)
Note: there can be more than one reactant in the reaction, not all first-order reactions have just one
reactant!
(Half-life - the time it takes for half of a sample to react - is the most common first order reaction)
Hons Chemistry
4
The General Rate Law
rate = k [A]™ [B]"
k is a constant, [A] and [B] are the concentrations of reactants
A and B. The exponents m and n are the reaction orders.
The rate of a reaction is the product of k and the concentrations of the reactants each raised to a power
(reaction order). The numbers m and n are determined experimentally and cannot be inferred from a
balanced equation. This is done by varying the concentration of each reactant in turn as the
concentrations of the others are held constant.
7
Example:
2 NO (g) + 2 H2 (g) > N2 (g) + 2 H2 (g)
Rate = k [NO]2 [H2]
If H2 is doubled, the rate doubles
If NO is doubled, the rate quadruples because 22 = 4
First-order in H2, second-order in NO, third-order overall
(the overall order of a reaction is the sum of the exponents in the rate law)
[ ]2
2
[ ]
Rz
R
1
The method of initial rates determines reaction order by comparing the initial rates of a reaction carried
out with varying reactant concentrations.
a
Experimental initial rates for:
a A + b B > products
| Initial [A] /M | Initial [B] /M | Initial Rate / mol/L s | |
|---|---|---|---|
| Trial 1 | 0.100 | 0.100 | 2.00 x 10-3 |
| Trial 2 | 0.200 | 0.100 | 4.00 x 10-3 |
| Trial 3 | 0.200 | 0.200 | 16.00 x 10-3 |
Compare trials 2 and 1: ([A]2 / [A]1)} = R(A2) / R(A1)
(0.2 / 0.1)}=4x 10-3 /2x 10-3
so x = 1, and A is first order.
Compare trials 3 and 2: ([B]3 / [B]2)y = R(B3) / R(B2)
(0.2 / 0.1)Y = 16 x 10-3 / 4x 10-3
so y = 2, and B is second order.
Rate = k [A] [B]2
Hons Chemistry
[A]
[A]
=
R2
K
5
Rate Law Problems
1. What is the overall reaction order of the following reaction?
Rate = k [A]2 [B]2
2+ 2 = 4
2. In the following reaction, what is the overall reaction order if doubling [A] results in quadrupling the
reaction rate and doubling [B] results in a reaction rate eight times faster?
Rate = k [A]™ [B]"
([A]2 / [A]1)™ = R(A2) / R(A1)
(2 / 1)m = 4 / 1
so m = 2, and A is second order.
([B]2 / [B]1)" = R(B2) / R(B1)
(2 / 1)" = 8 / 1
so n = 3, and B is third order.
Overall order is 2 + 3 = 5
3. Deduce the rate law and the overall reaction order given the following data:
NH4+ (aq) + NO2 (aq) > N2 (g) + 2 H2O (1)
| Initial [NH4+] /M | Initial [NO2 ] /M | Initial Rate / mol/L s | |
|---|---|---|---|
| Trial 1 | 0.100 | 0.0050 | 1.35 x 10-7 |
| Trial 2 | 0.100 | 0.0100 | 2.70 x 10-7 |
| Trial 3 | 0.200 | 0.0100 | 5.40 x 10-7 |
NO2: Compare trials 2 and 1
(0.01 / 0.05)} = 2.70 x 10-7 / 1.35 x 10-7 so x = 1, and NO2 is first order.
NH4+: Compare trials 3 and 2
(0.2 / 0.1)y = 5.40 x 10-7 / 2.70 x 10-7
so y = 1, and NH4+ is first order.
Rate = k [NH4+] [NO2 ]. Overall is 1 + 1 = second order.
Hons Chemistry
6
INSTANTANEOUS REACTION RATES AND REACTION
MECHANISMS
2 H2O2 (aq) -> 2 H2O (1) + O2 (g)
The instantaneous rate is the slope
of the straight line tangent to the
curve at the specific time.
Instantaneous reaction rate can be calculated
using the rate law:
Change in [H2O2] with Time
1.00
A [H2O2]
Instantaneous rate =
At
0.80
[H2O2] (mol/L)
0.60
0.40
A [H2O2]
0.20
At
0
0 1 2 3 4 5 6 7 8 9 10
Relative time (s)
2 N2O5 (g) > 4 NO2 (g) + O2 (g)
Rate = k [N2O5]
If k = 1.0 x 10-5 s-1 and [N2O5] = 0.350 mol/L
Rate = (1.0 x 10-5 s-1) (0.350 mol/L) = 3.5 x 106 mol/(Les)
Problem:
The following reaction is first order in H2 and second order in NO with k = 2.90 x 102 L2/ mol2.s
2 NO (g) + H2 (g)-> N2O (g) + H2O (g)
Calculate the instantaneous rate when [NO] = 0.00200 M and [H2] = 0.00400 M
Rate = k [NO]2 [H2]
Rate = 2.90 x 102 (0.002)2 (0.004) = 4.64 x 106 mol/(Los)
Hons Chemistry
7
REACTION MECHANISMS
Most chemical reactions consist of sequences of two or more simpler reactions. Each step is called an
elementary step.
A complex reaction contains two or more elementary steps.
A reaction mechanism is the complete sequence of elementary steps that makes up a complex reaction.
activated
complexes
internediates
Producty
Roaction progress
The slowest elementary step in a complex reaction is called the rate-determining step. It is the step with
the highest peak energy, so in the diagram above it is the second step. The complex reaction, above, has
four steps. To clarify, the RDS has the highest energy, not necessarily the largest individual hump.
An intermediate is a substance produced in one of the elementary steps and consumed in a subsequent
elementary step. Intermediates do not appear in the net chemical equation.
A catalyst may be a molecule that reacted in one step and is reformed in a subsequent step.
Hons Chemistry
8
CHEMICAL EQUILIBRIUM
Chemical equilibrium occurs when the forward and reverse reaction are taking place at the same rate.
In a chemical equation, the following symbol
1
is used to separate the reactants and products, and
shows the reversibility instead of a reaction. (It is used instead of >).
e.g. H2 + 2
1L
₹ 2 HI
the products may react back to the original reactants
Other examples of reversible reactions
- rechargeable batteries
- solubility ... dissolving and precipitating happening at the same time
Monitoring a reaction that reaches equilibrium
A + B
C + D
7
[C] and [D]
Conc
@gm established,
Concentrations
are constant
[A] and [B]
Time
Hons Chemistry
9
At equilibrium, the rates of the forward
and reverse reactions are equal
The concentrations of reactants and products
are constant.
The forward and reverse reactions continue
after equilibrium is attained.
Equilibrium Characteristics
1. Closed container (nothing leaving or
entering)
2. Temperature must remain constant
3. All reactants and products are present
and in constant dynamic motion.
4. AG = 0
5. A system at equilibrium is a stable system
Rate decreasing with time
A + B - C +D
Reaction rates
Equilibrium
constant
reactionrates
C + D - A + B
\Rate increasing with time
Time
This reaction is a homogeneous equilibrium, which means that all the reactants and products are in the
same physical state.
H2 (g) + I2 (g)
1L
2 HI (g)
When the reactants and products are present in more than one physical state, the equilibrium is called a
heterogeneous equilibrium.
e.g. vapor pressure of ethanol in a closed flask:
C2H5OH (l)
1
C2H5OH (g)
a
Hons Chemistry
10