Amino Acids and Protein Structure: Biochemistry Essentials
Slides from Biocd 1556 about Amino Acids and Protein Structure. The Pdf explores the structure of amino acids and proteins, fundamental to biochemistry, covering protein structure levels and their health implications. This University Biology material includes learning objectives, keywords, and suggested readings.
See more17 Pages

Unlock the full PDF for free
Sign up to get full access to the document and start transforming it with AI.
Preview
Amino Acids and Protein Structure
BIOCD 1556, 2025 Lecture 2 Dr. Jonathan Lerner Amino Acids and Protein Structure Introduction Mammalian proteins are comprised of 20 amino acids, each of which has unique biochemical properties that can influence the structure and function of proteins. Proteins have widely diverse roles in biology, and their linear amino acid primary structures don't tell the whole story. Individual bonds between amino acids and proteins (i.e., peptide, hydrogen, ionic, disulfide bonds and hydrophobic and hydrophilic interactions) influence the shape and nature of a protein at physiological pH. Altering a protein's amino acid sequence or folding can greatly influence a protein's function and have negative effects on an individual's health. Many genetic diseases result in proteins with abnormal primary amino acid sequences. Gaining an appreciation for the basic characteristics of amino acids and the levels of protein structure are critical to the understanding of biochemical processes at play in human health.
Terminal objective: To understand how the structures and properties of amino acids determine the properties, structure and function of proteins.
Enabling Objectives
- Classify amino acids into appropriate groups based upon the structure and properties of their unique R-group side chains.
- Apply knowledge of the properties of amino acids to understand the structures, properties, and functions of proteins.
- Define protein primary, secondary, tertiary, and quaternary structures.
- Describe the types and nature of bonds and forces that stabilize primary, secondary, tertiary, and quaternary protein structure.
- Predict how changes to a protein's amino acid primary structure may alter its final structure, properties, and function.
Key Words
Amino Acid Structure
pH, pK, Ca, carboxyl and amine groups, side chains properties: non-polar, polar + uncharged, polar + basic, polar + acidic
Protein Structure
primary structure: peptide bonding, N- and C- terminal end, peptide, protein; secondary structure: alpha-helix, beta-strand, beta-sheet, beta-turn, motifs, non-repetitive; tertiary structure: disulfide bonds, hydrogen bonds, hydrophobic and ionic interactions, folding and chaperones, protein domains; quaternary structure
Implications for Human Health
Amyloids, HbS, Recombinant Insulin Glargine
Suggested Reading
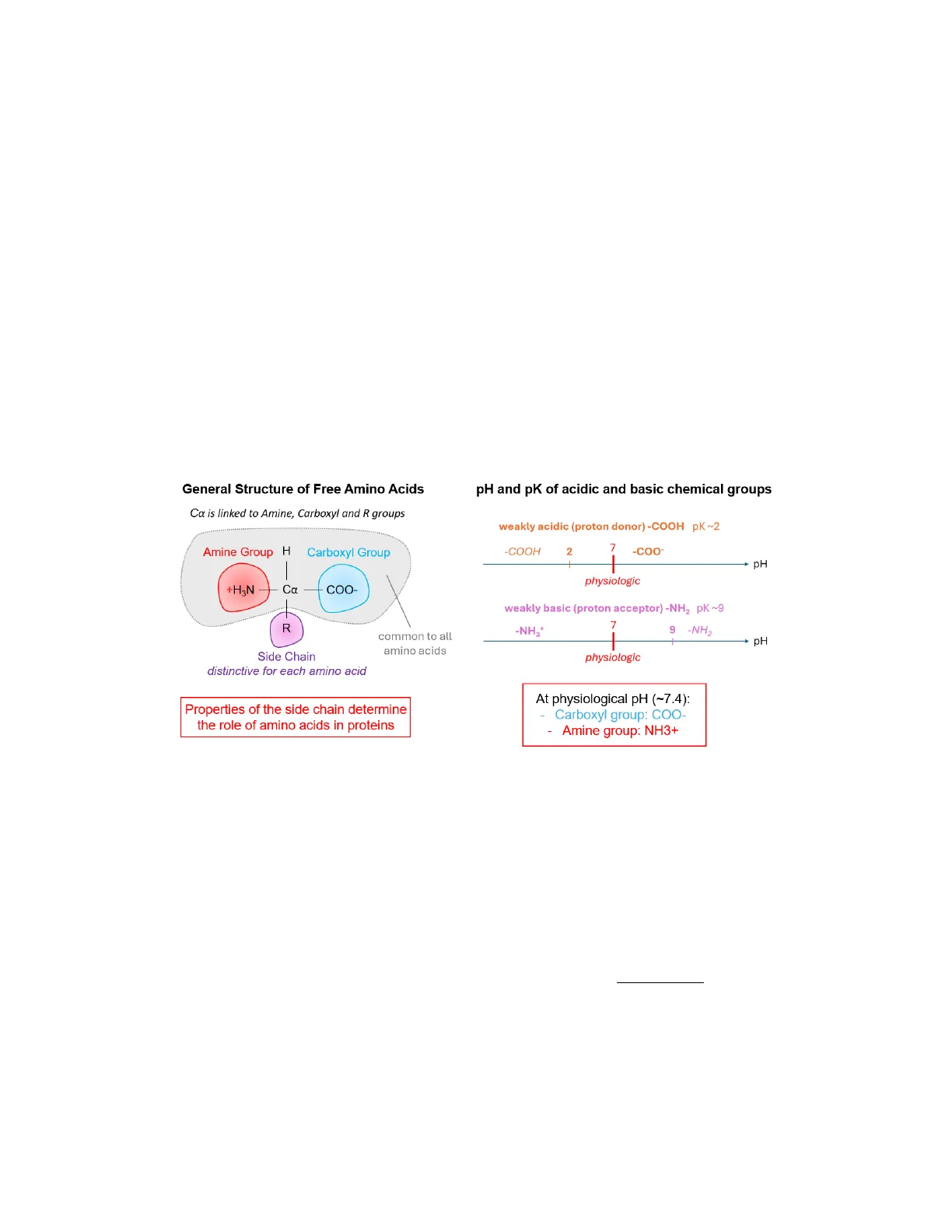
Lippincott's ILLUSTRATED REVIEW OF BIOCHEMISTRY, 8th edition, by Abali, Cline, Franklin and Viselli (2021) Chapter 1: 1-6; Chapter 2.Part 1. Amino Acid Structure 20 amino acids (residues) are found in mammalian proteins. Each has a:
- central carbon atom: Ca
- carboxyl group: - COOH (pK ~2.3)
- primary amine group: - NH2 (pK ~9.1)
- hydrogen atom
- distinctive side chain: R-group
At the physiological pH (~7.4):
- the carboxyl group loses a H+ to form a negatively charged carboxylate ion (-COO-)
- the amine group captures a H+ to form a positively charged amine group (-NH3+) The properties of the R-group side chain of an amino acid determine the amino acid's role in a protein.
General Structure of Free Amino Acids
Ca is linked to Amine, Carboxyl and R groups Amine Group H Carboxyl Group +H3N Ca COO- R common to all amino acids Side Chain distinctive for each amino acid Properties of the side chain determine the role of amino acids in proteins
pH and pK of Acidic and Basic Chemical Groups
weakly acidic (proton donor) -COOH pK ~2 -COOH 2 7 -COO- + pH physiologic weakly basic (proton acceptor) -NH2 PK ~9 7 -NH3+ 9 -NH2 -PH physiologic At physiological pH (~7.4):
- Carboxyl group: COO-
- Amine group: NH3+ General structure, pH and pK of Amino Acids N.B .: The single R-group of amino acids makes them asymmetric and able to exist in two mirror image forms (D and L). Only L-amino acids are found in mammalian proteins, and D-amino acids are minimal constituents of living organisms.
Amino Acids with Non-polar/Hydrophobic Side Chains
Apolar side chains are made of Carbons and Hydrogens and are hydrophobic (= non-polar = apolar). As non-polar side chains are also uncharged, these amino acids are Zwitterionic: neutrally charged molecules that contain positive (-NH3+) and negative (-COO) charges at physiological pH. In aqueous, polar environments, non-polar R-groups cluster in the interior of a protein due to hydrophobic interactions.In hydrophobic environments, such as the plasma membrane, R-groups arrange at the exterior of a protein in contact with the lipid environment. Amino acids with non-polar sidechains include:
- Glycine: smallest R-group (H), low steric hindrance
- Proline: unique for its secondary amine group. The Nitrogen atom forms a bond with Ca to form a rigid, five-membered ring structure.
- Branched Chains Amino Acids (BCAAs): Valine, Leucine, Isoleucine (see lecture on protein metabolism)
- Alanine
- Methionine, which contains a Sulfur atom.
- Aromatic, cyclic amino acids Phenylalanine and Tryptophan. NONPOLAR SIDE CHAINS COOH -pK1= 2.3 COOH COOH +H3N-C-H +H3N-C-H *H3N-C-H - H CH3 CH pK2= 9.6 H3C CH3 Glycine Alanine Valine COOH COOH COOH *H3N-C-H +H3N-C-H +H3N-C-H CH2 H-C-CH3 CH2 CH CH2 H3C CH3 CH3 Leucine Isoleucine Phenylalanine COOH COOH +H3N-C-H +H3N-C-H COOH CH2 CH2 +H2N-C-H 1 C CH2 H2C CH2 CH2 CH 15- N H CH3 Tryptophan Methionine Proline Amino Acids with non-polar side chains
Amino Acids with Uncharged and Polar/Hydrophilic Side Chains
Amino acids with uncharged polar side chains are:
- Zwitterionic
- Establishing hydrogen bonds (see Part 2C): the hydrophilic nature of the side chain is conferred in part by their ability to establish hydrogen bonds with water molecules. Amino acids with uncharged and polar sidechains include:
- Serine, Threonine and Tyrosine (the third aromatic amino acid): present a hydroxyl (-OH) group that is a prime target for post-translational modifications: o Phosphorylation by kinase enzymes - removed by phosphatase o Other: O-linked glycosylation, Sulfation, ADP-ribosylation, ...- Cysteine, which side chain contains a sulfhydryl (-SH) group which can participate in dimerized covalent cross-links to form disulfide bonds (-S-S-) with another Cysteine residue (see Part 2C).
- Asparagine and Glutamine, which sidechains contain an amide group (-CONH2). N.B .: Despite the -NH2, amide groups present a neutral charge at physiological pH, due to a resonance between the C and N atom, decreasing the basicity of the latter (pK~20). UNCHARGED POLAR SIDE CHAINS COOH - PK1 =2.2 +H3N-C-H COOH COOH CH2 pK2= 9.1 H-C-OH H -C-OH CH3 OH- PK3= 10.1 Tyrosine Serine COOH Threonine COOH +H3N-C-H +H3N-C-H COOH -PK1= 1.7 +H3N-C-H CH2 pK3= 10.8 SH-PK2=8.3 O NH2 Asparagine Glutamine Cysteine Amino Acids with uncharged polar side chains
Amino Acids with Negatively Charged (Anionic/Acidic) Polar Side Chains
Aspartic Acid and Glutamic Acid contain carboxylic acid groups (-COOH) in their side chains, which donate protons (H+) at physiological pH to form fully ionized, negatively charged carboxylate groups (-COO), also known as Aspartate and Glutamate. ACIDIC SIDE CHAINS pK1 = 2.1 COOH COOH pK3=9.8-++H3N-C-H pK3=9.7-++H3N-C-H CH2 CH2 C. CH2 O OH-PK2= 3.9 C OH -pK2= 4.3 Aspartic acid Glutamic acid Amino Acids with negatively charged side chains
Amino Acids with Positively Charged (Cationic/Basic) Polar Side Chains
Amino acids with positively charged side chains include:
- Lysine, which side chain ends up with a primary amine group (-NH2), fully ionized and positively charged at physiological pH (-NH3+).
- Arginine, which side chain contains a guanidium group (-HN=C(NH2) 2), which is fully ionized and positively charged at physiological pH (-HN=C(NH2) 2+).
- Histidine, which side chain is a weakly basic imidazole ring. When Histidine is incorporated into a peptide or a protein, its side chain can be positively charged or neutral depending upon the ionic environment of the surrounding polypeptide chains. +H3N-C-H +H3N-C-H I -0-I CH2 CH2 C CH2 O NH2 CBASIC SIDE CHAINS pK1 = 1.8 pK1 = 2.2 pK3 =9.2 pK2 = 9.2 pK2= 9.0 COOH COOH COOH +H3N-C-H +H3N-C-H +H3N-C-H Amino Acids with positively charged side chains
Amino Acid Nomenclature
By convention, Amino Acids can be named:
- by a three-letter code matching the first three letters, with some exceptions: Ile, Trp, Asn, ...
- by a single letter code matching the first letter, with some exceptions: F, W, Y, N, ... hydrophobic Hydrophilic/polar neutral charge neutral charge positive charge negative charge Glycine Gly G Serine Ser S Lysine Lys K Aspartic Acid Asp D Proline Pro P Threonine Thr T Arginine Arg R Glutamic Acid Glu E Valine Val V Tyrosine Tyr Y Histidine His H Leucine Leu L Cysteine Cys C Isoleucine lle I Asparagine Asn N Alanine Ala A Glutamine Gln Q Methionine Met M Phenylalanine Phe F Tryptophan Trp W
Protein Structure
In proteins, different combinations of the 20 amino acids are linked together by peptide bonds. The linear sequence of amino acids contains all the information required to generate a protein with the correct three-dimensional shape and biochemical properties.
Primary Structure
The primary structure of a protein is the linear sequence of amino acids linked by peptide bonds - encoded by DNA in the genome of an organism (see lecture on Transcription and Translation).
Peptide (Amide) Bonding
Peptide bonds are covalent linkages between the a-carboxyl group of one amino acid and the a- amino group of another during translation. Peptides bonds are:
- rigid and planar, due to partial double-bond, and generally in the trans-configuration. CH2 CH2 CH2 C =CH CH2 CH2 +HNNH C CH2 CH2 1 H CH2 N-H pK2= 6.0 NH3+-PK3= 10.5 C=NH2+- PK3= 12.5 - NH2 Histidine Lysine Arginine- neutrally charged
- polar/hydrophilic, since the -NH and -C=O groups can participate in hydrogen bonding (see Part 2C). Formation of the peptide bond during Protein synthesis (translation) H H +H3N - Ca - COO- +H3N - - Ca - COO- R R H2O H H H +H3N- Ca C - N Ca - COO- O R peptide bond trans-configuration Ca N Ca H most common avoids steric "clashes" Peptide bonds cis-configuration Ca Ca C Z O H found mainly at X-Pro bonds enable tight turns A polypeptide chain contains a free amino group (N-terminal end) and a free carboxyl group (C- terminal end). By convention, polypeptide sequences are oriented from the N to C-terminal end. A short assembly of amino acids (<50 AAs) is a peptide, proteins are typically larger assemblies (50- 1000s of AAs). The N-terminal a-amine group (-NH3+) and C-terminal a-carboxyl group (-COO ) are charged. Thus, in the absence of amino acids with positively or negatively charged side chains, a protein will have a neutral charge at physiological pH. Peptide (2-50 AAs) Protein (50+ AAs) H H H H H H H I 1 I +H3N- Ca C - N Ca C -N - Ca -C -N -Ca COO- 1 N-terminal O R R 0 R O R peptide bond
Secondary Structure
The secondary structure of a protein consists in non-random, regular repeating periodic arrangements of amino acids near each other in a linear amino acid sequence.
Alpha-Helix
An a-helix is a single polypeptide motif with a spiral structure: a tightly packed, coiled polypeptide core with side chains extending outward to avoid steric interference. N- and C-terminal ends I -Z 1 C-terminal R