Isotonicity and Freezing Point Depression, University of Portsmouth Presentation
Slides from University of Portsmouth about Isotonicity and Freezing Point Depression. The Pdf explores colligative properties, osmotic pressure, and freezing point, with detailed tables on fluid composition and osmolarity, and an example calculation for isotonic concentration. This University Chemistry document is from 2024.
See more12 Pages

Unlock the full PDF for free
Sign up to get full access to the document and start transforming it with AI.
Preview
Colligative Properties of Solutions
The physical properties of a solution may vary with the concentration of the dissolved solutes (non-volatile).
- COLLIGATIVE if change in property is:
- Proportional to the concentration of the dissolved solute
- Independent of the nature of the solute
- Some molecules such as electrolytes can dissociate in water generating ions. This will influence their colligative properties as they depend on the individual species present in solution.
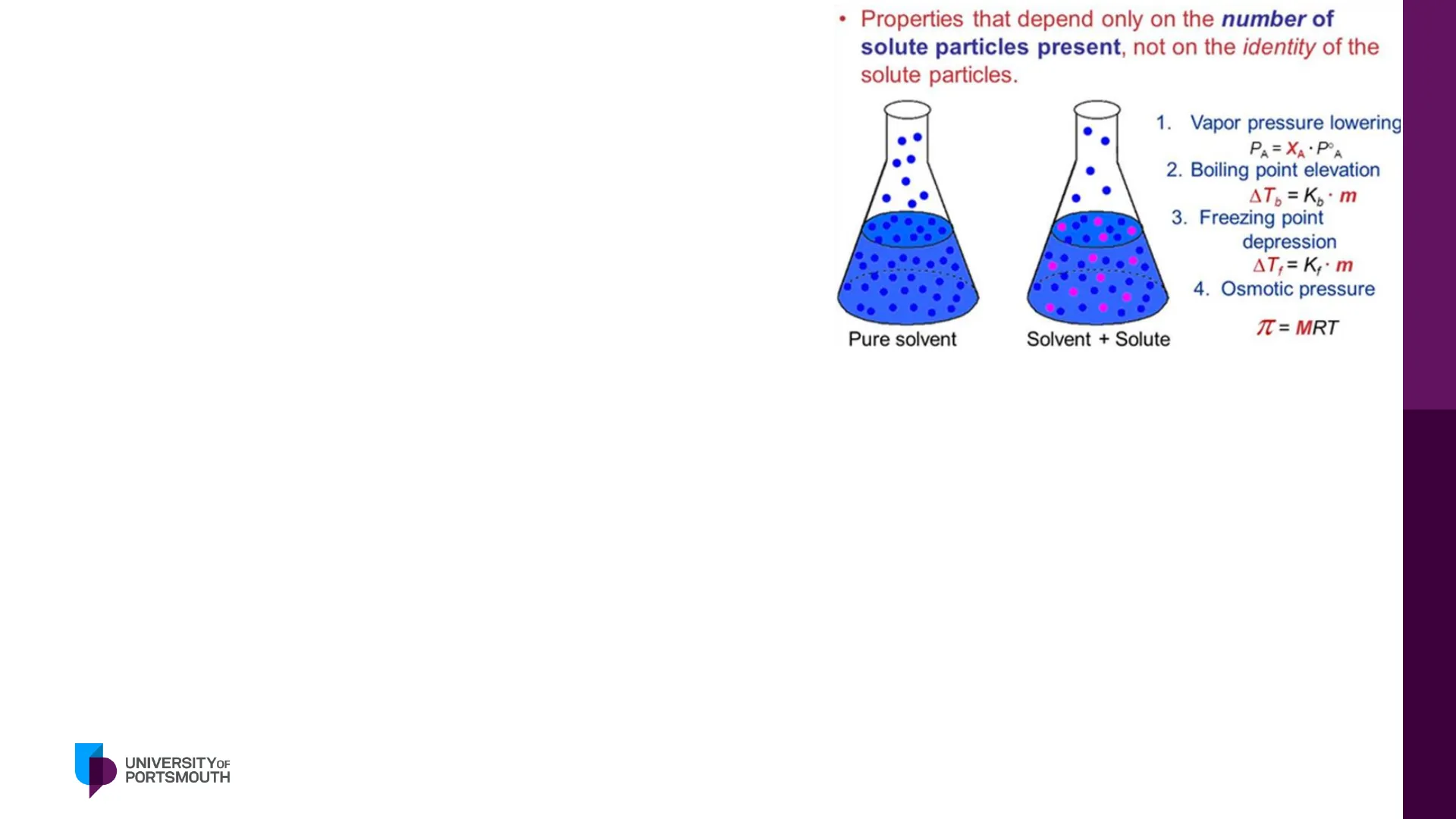
Properties that depend only on the number of solute particles present, not on the identity of the solute particles.
Key Colligative Properties
Pure solvent Solvent + Solute 1. Vapor pressure lowering PA = XA · POA 2. Boiling point elevation AT = Kp . m 3. Freezing point depression AT,= K,. m 4. Osmotic pressure T = MRT
Colligative properties are properties of solutions that change in relation to the number of solute particles present in the solvent. [We referred to colligative properties last year when we talked about ways in which we could measure the critical micellar concentration. Here we are thinking about solutions that contain drugs and other excipients. Some of these molecules in water will be able to dissociate creating ions. This is important as colligative properties depend on the individual species present in solution so we need to consider ionisation. When we have dissolved species in a solvent the vapour pressure, boiling point, freezing point and osmotic pressure will change. We are particularly interested in the osmotic pressure and freezing point as we will see in the next slides.
Osmotic Pressure
Pure water Solution Glucose Osmotic pressure, II H2O Semipermeable membrane (a) Initial state (b) Equilibrium
Osmolality and Osmolarity
Osmolality is the concentration (Osm/Kg) of a substance dissolved in a mass of solvent that will provide the same osmotic pressure as 1 mole of an undissociated molecule in 1 Kg of water.
Osmolarity (Osm/L) is the concentration (osmoles) of a substance dissolved in a volume of solvent that will provide the same osmotic pressure as 1 mole of an undissociated molecule in 1 litre of water.
OIf a molecule does not dissociate creating new species in water, 1 mole/ litre will be equal to 1 Osm/L. However when a molecule dissociates in water and gives rise to two or more species, the Osmolarity will depend on the number of species generated (regardless of the nature or the charge).
Dissociation Examples and Van't Hoff Factor
Dextrose - no dissociation i = 1 0.5 Mol/L = 0.5 Osmol/L NaCI -> Na++ Cl- i = 2 0.5 Mol/L = 0.5 x 2 = 1 Osmol/L Na2CO3 > 2Na++ CO32- i= 3 0.5 Mol/L = 0.5 x 3 = 1.5 Osmol/L i = Van't Hoff correction factor, is the number of species generated from dissolution of the parent molecule.
Osmotic Pressure and Isotonicity of Pharmaceutical Products
Biological membranes (e.g., cell membrane of erythrocytes) behave as semipermeable membranes.
Parenteral formulations such as injections and ophthalmic preparations must be isotonic with (have the same osmolarity as) the body fluids they mix with.
- IV - isotonic to serum
- Intrathecal - isotonic to CSF
- Intraocular - isotonic to vitreous humour
The osmolarity of a solution also dictates if it can be injected or infused and at what rate.
Why do we need to know about osmolarity? Because the value of osmolarity of a solution compared to the osmolarity value of the tissue into which that solution will be injected, will tell us if a solution is isotonic or not. The osmolarity of a solution will also tell us if it can be injected or infused and at what rate. Depending on the site of administration we need to prepare solutions that are isotonic with the fluids they are injected to, so if IV the solution must be isotonic to serum, if intraocular it must be isotonic with the vitreous humour, and so on.
Tonicity Classifications
Hypertonic Isotonic Hypotonic Crenation UNIVERSIT Reversible) PORTSMOUTH >295 mOsm/L H2O H2O H2O H2O 275-295 mOsm/L <275 mOsm/L Haemolysis (Irreversible)
Hypertonic Solutions: Total Parenteral Nutrition (TPN)
Hypertonic solutions may be used " when rapidly diluted by the blood e.g. administration via central intravenous catheter (central line - subclavian or jugular vein access to vena cava) Slow infusion >550 mOsmol/L
Applications of Hypertonic Solutions
- Comatose patients (ICU)
- Enteral feeding not possible
- Chemotherapy patients (short term)
There are cases in which hypertonic solutions can be used as they will undergo a degree of dilution once injected. They are suitable for injection via a central line as they join a blood vessel with high quantity of blood. They should always be infused at low rate.
TPN Osmolality Examples
TPN Pump Catheter Solution Tonicity (mosmol kg 1) Vamin 9 700 Vamin 9 Glucose 1350 Vamin 14 1145 Vamin 14 Electrolyte-free 810 Vamin 18 Electrolyte-free 1130 Vaminolact 510 Vitrimix KV 1130 Intralipid 10% Novum 300 Intralipid 20% 350 Intralipid 30% 310 Intrafusin 22 1400 Hyperamine 30 1450 Gelofusine 279ª Lipofundin MCT/LCT 10% 345ª Lipofundin MCT/LCT 20% 380ª Nutriflex 32 1140ª Nutriflex 48 1400ª Nutriflex 70 2100ª Sodium Bicarbonate Intravenous Infusion BP 8.4% w/v 2000ª 4.2% w/v 1000ª ª Osmolarity (mosmol dm-3).
Tonicity must be adjusted e.g. by addition of salt, dextrose, or mannitol. As the osmotic pressure is difficult to measure to adjust the tonicity of a solution we use the measurement of a correlated colligative property: freezing point depression Serum freezes at -0.52 ℃, therefore any product with a FP = - 0.52 ℃ will be isotonic with serum.
Freezing Point and Tonicity
Hypotonic · 0 ℃ e.g. solution with a freezing point of - 0.4℃ is hypotonic Hypotonic HO - 0.52 °C Isotonic e.g. solution with a freezing point of -1.0℃ is hypertonic Hypertonic Hypertonic - Infinity H,O
Composition & Osmolarity of Commonly Used Fluids
Composition of commonly used crystalloids Content Plasma Sodium chloride 0.9%* Sodium chloride 0.18%/ 4% glucoseª 0.45% NaCI/ 4% glucoseª 5% glucoseª Hartmann's Lactated Ringer's (USP) Ringer's acetate Alternative balanced solutions for resuscitation ** Alternative balanced solutions for maintenance ** Na+ (mmol/l) 135-145 154 31 77 0 131 130 130 140 40 Cl- (mmol/l) 95-105 154 31 77 0 111 109 112 98 40 [Na]:[CH-] ratio 1.28-1.45:1 1:1 1:1 1:1 1.18:1 1.19:1 1.16:1 1.43:1 1:1 K+ (mmol/l) 3.5-5.3 . * * . 5 4 5 5 13 HCO3 - 1 Bicarbonate 24-32 0 0 28 (lactate) 27 (acetate) 27 (acetate) 23 (gluconate) 16 (acetate) Ca2+ (mmol/l) 2.2-2.6 0 0 0 2 1.4 1 0 0 O Mg2+ (mmol/l) 0.8-1.2 0 0 0 0 1 1.5 1.5 Glucose (mmol/ l) 3.5-5.5 0 222 (40 g) 222 (40 g) 278 (50 g) 0 0 0 0 222 (40 g) pH 7.35-7.45 4.5-7.0 4.5 3.5-5.5 5.0-7.0 6-7.5 6-8 4.0-8.0 4.5-7.0 Osmolarity (mOsm/l) 275-295 308 284 278 278 273 276 295 389 " These solutions we available with differing quantities of potassium already added, and the potassium-containing versions are usually more appropriate for meeting maintenance needs. "Alternative balanced solutions are available commercially under different brand names and composition may vary by preparation. "The term dextrose refers to the dextro-rotatory isomer of glucose that can be metabolised and is the only form used in IV fluids. However IV fluid bags are often labelled as glucose so only this term should be used. Traditionally hospitals bought a small range of fluids combining saline (0.18-0.9%) with glucose but several recent NICE/NPSA documents have recommended specific combinations, which are now purchased to enable guidelines to be followed. Glucose-saline combinations now come in 5 different concentrations, and the addition of variable potassium content expands the pre-mixed range to 13 different products. Prescribers must therefore specify the concentration of each component; the term dextrose-saline (or abbreviation D/S) is meaningless without these details. What is specified also impacts significantly on the cost of the product. Note: Weight-based potassium prescriptions should be rounded to the nearest common fluids available (for example, a 67 kg person should have fluids containing 20 mmol and 40 mmol of potassium in a 24-hour period). Potassium should not be added to intravenous fluid bags as this is dangerous. Source: This table was drafted based on the consensus decision of the members of the Guideline Development Group. 'Intravenous fluid therapy in adults in hospital', NICE clinical guideline 174 (December 2013. Last update December 2016) In this table you have the values of osmolarity of common fluids used for parenteral administration.
Freezing Point Depression
OTo prepare a solutions that is isotonic with blood we need to prepare a solution that freeze at - 0.52℃. OFreezing point depression value indicate change to the freezing point from that of water for a 1% w/v solution of any given substance. OIsotonic agent " Chemically and physically compatible with the formulation Non-toxic and biocompatible Sufficient quantity to depress the freezing point of water by 0.52℃ (as of plasma) The Pharmaceutical Codex
DFD2 - Freeze Point Depression Values
Prednisolone sodium phosphate 0.51° Benzalkonium chloride 0.091° Sodium chloride 0.576° Sodium metabisulphite 0.389º Potassium acid phosphate 0.252° Sodium phosphate dibasic (12H2O) 0.126° Phentolamine mesylate 0.096° Glucose (anhydrous) 0.100° Chlorpromazine hydrochloride 0.058° Ascorbic acid 0.105°
Freezing Point Depression Example
Example · FP of 1% w/v NaCI = - 0.576℃ · FP serum = - 0.52℃ Hence 1 %w /v - 0.576 ℃ ~- 0.52 ℃ X %w /v x = 0.902 %w/v concentration of sodium chloride solution which is isotonic ONote. Discrepancy due to incomplete dissociation: · From osmolarity calculation : 0.847% · From freezing point calculation: 0.902% · From biological studies: 0.9%
Freezing Point Depression Calculation Question
Question: Calculate the amount of sodium chloride required to adjust the tonicity of 100 ml of 1% w/v lignocaine hydrochloride injection (FP of 1% w/v lignocaine hydrochloride = - 0.13ºC). (FP of 1% w/v NaCI = - 0.576°℃). Answer: [FP dep. due to lignocaine] + [FP dep. due to NaCI] = 0.52ºC (0.13ºC × 1% w/v) + (0.576°℃ x X% w/v) = 0.52ºC 0.13 + 0.576 X = 0.52 X = 0.677% w/v NaCl concentration = 0.677 g (677 mg) NaCl in 100 ml