Memoria ed esaurimento delle cellule T: differenziazione e sopravvivenza
Slide di Università sulla memoria ed esaurimento delle cellule T. Il Pdf esplora la differenziazione delle cellule T naive in cellule della memoria, i requisiti per la loro sopravvivenza e la generazione di memoria dopo un'infezione, con modelli di differenziazione e esperimenti chiave per Biologia.
Mostra di più13 pagine

Visualizza gratis il Pdf completo
Registrati per accedere all’intero documento e trasformarlo con l’AI.
Anteprima
Meccanismi di base delle malattie: Memoria ed esaurimento delle cellule T
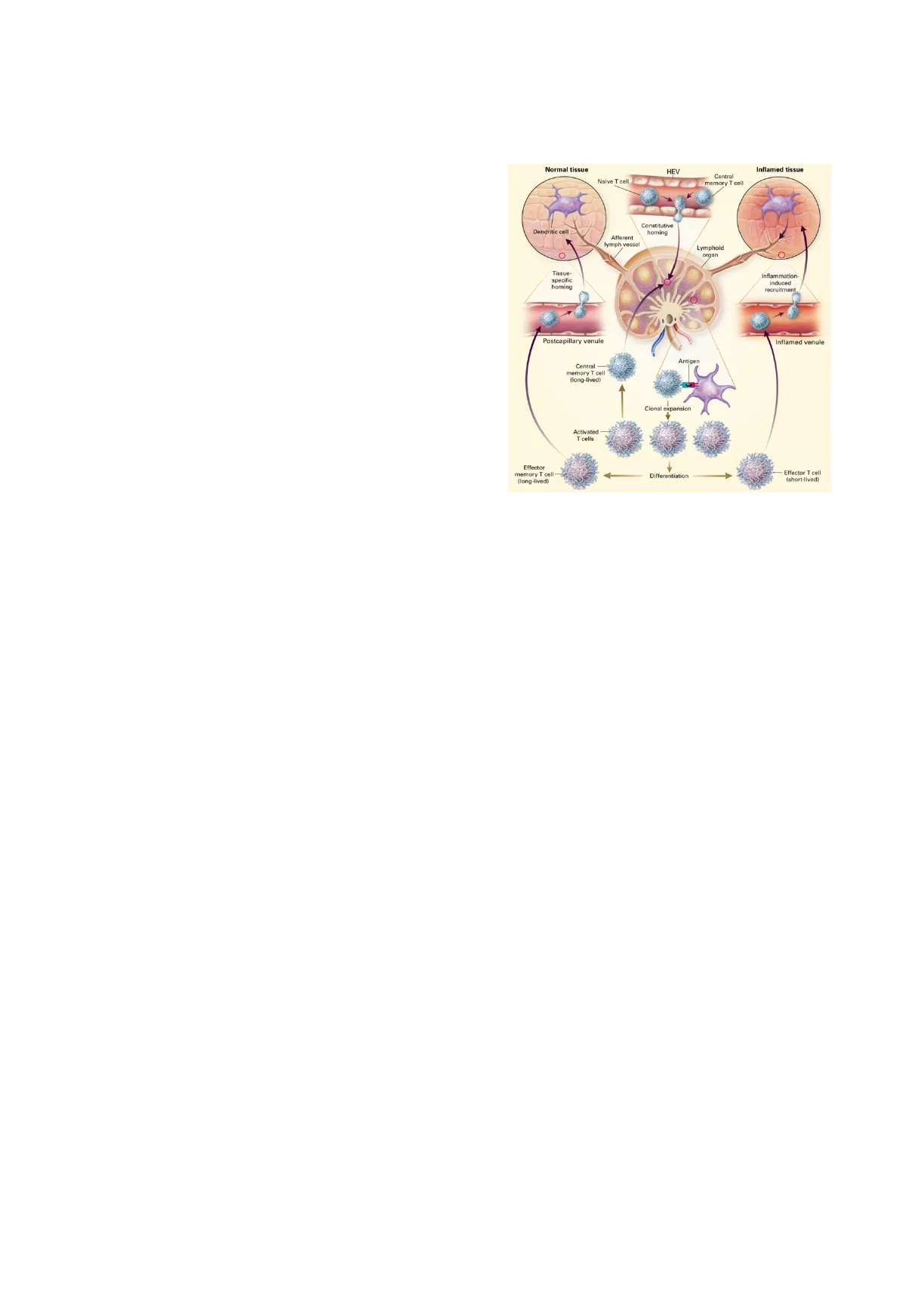
Author: Lorenzo Panduri Prof. Matteo Iannacone Basic Mechanisms of Diseases, MI5 04/12/2023 T cell memory and exhaustion In the blood, naïve T cells home to secondary lymphoid organs via specialized vessels within the T cell area of the lymph node, which are made of high endothelial venules (HEVs). HEVs can provide the ligands for certain receptors present on naïve T cells that allow them to home to lymph nodes. Receptors on naïve T cells are CD62L (L-selectin), CCR7, and LFE1.
When naïve T cells migrate out of the vessel, they enter the lymph nodes, where they maintain the interstitial motility of about 10 microns per minute. Naïve T cells scan the lymph node for the presence of specific antigens that can be recognized on the surface of specialized antigen presenting cells, referred to as dendritic cells.
Normal tissue HEV Inflamed tissue Naive T cell Central memory T cell 00 Constitutive homing Dendritic cell Afferent lymph vessel Lymphoid organ O Tissue- specific homing Inflammation- induced recruitment Postcapillary venule Inflamed venule Antigen Central memory T cell (long-lived) Clonal expansion Activated T cells Effector memory T cell (long-lived) Differentiation Effector T cell (short-lived)
If naïve T cells do not find the antigen, they will leave the lymph nodes via the efferent lymph vessels, going to the thoracic ducts, subclavian vein, back into the systemic circulation, and, finally, they will go to another lymph node.
Instead, if they do find and recognize the antigen on dendritic cells via their pattern recognition receptors, they upregulate CCR7, migrate to lymph nodes, localize into the T cell area, and they are able to present the antigen to T cells: at this point MHC-I and MHC-II molecules will be able to activate CD4+ and CD8 T cells.
If that happens with a sufficiently high affinity recognition and in the presence of signal-2 (provided by CD28 co-stimulation) and cytokines, it will lead to clonal expansion: the same T cell will divide exponentially giving rise to millions of identical cells with the same receptor.
Moreover, T cells differentiate and acquire the effector function, for example, CD8 T cells become cytotoxic (killing antigen presenting cells) and produce cytokines, such as interferon-gamma.
After a couple of weeks, most of these cells will die as the response contracts, but a few cells, called memory cells, will survive to will survive to create a long-time memory ready to be activated much easier later in comparison to naïve T cells.
Memoria immunitaria
Memory is one of the hallmark characteristics of the adaptive immunity.
In the 19th century, a natural experiment was performed in the Faroe Islands: these islands were subjected to travel restrictions for a period, so they were completely isolated from the rest of Europe.
In 1781, there was a severe measles epidemic on Faroe Islands. However, between 1782 and 1845, no cases of measles were reported, because this was the period of embargo, and no one was allowed to travel in or out.
Page 1 of 13Author: Lorenzo Panduri Prof. Matteo Iannacone Basic Mechanisms of Diseases, MI5 04/12/2023 When the travel restrictions eased in 1846, there was a major epidemic of measles for which 75-95% of the population was affected.
In the same year, a medical student, called Peter Ludwig Panum, was sent to the Faroe Islands to investigate the epidemic. He observed that of the many aged people still living on the Faroes who contracted measles in 1781, not even one was attacked a second time.
Instead, all the old people who had not contracted measles in earlier life, were attacked when they were exposed to the infection.
This experiment showed that protective immunity could last for decades (in this case it lasted for 65 years) in absence of re-exposure. In today's lingo, this would be referred to as an antigen-independent immune memory, where a constant exposure to the pathogen is not needed to be protected.
Differenze tra cellule T naive e della memoria
When we observed the kinetics of the immune response, comparing the primary and secondary immune responses, there were some differences regarding the magnitude, the rapidity of onset and the length time: this is because there are more memory cells than naïve cells. In addition, there are intrinsic characteristics of memory cells that make them more capable to react to a secondary exposure.
In naïve T cells there are a lot of closed loci, so the chromatin is closed, and they are not easily accessible by transcription factors. Instead, in memory cells the chromatin is open, so the loci for a lot of effector genes are open.
Memory cells also have pre-existing transcription factors that are already synthesized within the cell, which, instead, are not present in naïve cells. These transcription factors make it such that as soon as they receive an activation signal, they immediately transcribe effector genes. Therefore, memory cells react faster than naïve cells, as they do not need to reorganize the chromatin, open the loci and produce transcription factors.
Moreover, there is no need of signal 2 to activate a memory cell, while naïve T cells need to receive a signal through the TCR, but a CD28 co-stimulation and higher cytokine production are also necessary.
Generazione di cellule T della memoria dopo un'infezione
To understand the half-life of T cells, in an experiment that was carried out in mice, the investigator tracked one T cell population that recognized one specific antigen by exploiting the use of MHC tetramers (major histocompatibility complex tetramers) - because TCRs recognize complexes of MHC and peptides.
To track single specificity in T cells, one trick is to link four MHC molecules with the respective peptide together, for example with biotin and streptavidin - streptavidin has tetra valency, meaning that it is capable of binding four different Biotins.
So, if you take MHC and peptides, conjugate them with biotin and mix them with streptavidin, you will end up with this tetramer. With this reagent, you can fish out even very rare T cells.
Page 2 of 13Author: Lorenzo Panduri Prof. Matteo lannacone Basic Mechanisms of Diseases, MI5 04/12/2023 By using specific enrichment techniques, you are also capable of seeing how many naive cells of a single specificity are present in a particular experimental animal.
With the same technique, the investigator was able to track the expansion and contraction of one single specificity.
Before immunization or infection, there are between 10^1 and 10^2 naïve T cells that are specific for a particular epitope.
After immunization, he observed an incredible expansion of memory T cells of more than 3 logs over a week - the cycle time of T cells is around 8 hours, so it is very fast.
LLOp:I-Ab+ 106 106 cells 105. 105 104- 104- 103- 103- 102 102- 101 10 0 2 4 6 8 10 0 50 100 150 200 250 300 350 400 450 Time after immunization (days)
The second graph shown on the right is also Half life in humans: 8-15 years about memory T cells, but it is expanded on a longer period of time, where we can appreciate that, considering that a mouse's life span is about 2 years, memory cells peak after one week, and within another week or two, 95% of the cells die. After that there is a very very slow decay, meaning that the populaiton remains almost constant or declines at a slow pace after the first weeks.
It is estimated that in humans the half-life of memory T cells is 8-15 years, which doesn't refer to a single memory T cell, but to the population of memory cells that have self-renewal capabilities.
Requisiti di sopravvivenza delle cellule T della memoria
Memory T cells require IL-7/IL-15 for survival When a T cell encounters an antigen on an antigen-presenting cell, with the presence of signal 2 and 3, most activated T cells become effector cells, killing target cells or producing cytokines, however, most effector cells are short-lived.
Some activated and/or effector cells can survive and become memory cells via the up-regulation of a particular cytokine receptor, which is IL-7 receptor: only the cells that will upregulate this receptor can sense IL-7, which will provide surviving signals for these cells.
In the absence of IL-7, effector cells will die.
Effector cells activate a transcriptional program by which pro-apoptotic molecules lead to their death. Nevertheless, such signal is counteracted by IL-7 and IL-15 signaling. IL-7 and IL-15 signal through the same receptor, and they are cytokines required for survival.
As previously discussed, Panum realized that antigen re-exposure is not needed for memory cells to persists and provide protective immunity, but thanks to several experiments done on animals, investigators discovered
Naive T cells require signals from contact with self peptide:self MHC complexes and the cytokines IL-15 and IL-7 for survival IL-7R AIL-7 V TCR self peptide- APC Naive T cell encounters antigen APC Most activated T cells become effector cells Some activated and/or effector cells become long-lived memory cells target cell Many effector cells are short lived and die by apoptosis Cytokines IL-7 and IL-15 are required for survival 3 V Memory T cells still need contact with peptide:self MHC complexes to actively proliferate TCR APC Page 3 of 13 L-7RAuthor: Lorenzo Panduri Prof. Matteo Iannacone Basic Mechanisms of Diseases, MI5 04/12/2023 that in addition to IL-7 and IL-15, memory cells need to contact MHC molecules that are presenting self-peptides.
As we previously studied, positive selection in the thymus results in a T cell repertoire that is specific for self-peptides.
T cells that have a TCR that recognize self-molecules to a high affinity will be removed from the repertoire via negative selection, because their activation would result in autoimmunity.
Positive selection is such that T cells react to MHC class I molecule to make sure that they recognize your own MHC: MHC complexes are always loaded with peptides, but in case the cell is not infected, the peptides contained are self-peptides.
What is important is that memory T cells need constant recognition to survive. If you remove an MHC class I from the host, then memory cell cannot survive.
In summary, homeostatic cytokines IL-7 and IL-15, recognition of MHC molecules and self- peptides are critical for survival.
Esperimento sulla sopravvivenza delle cellule T della memoria
Below it is shown an experiment carried out in mice that demonstrated that IL-7 is required for survival.
Tracking antigen-specific T cells, at the peak of primary response most cells, being IL- 7Ralo cells, become effector cells, while only a few cells will upregulate IL-7Rghi. If we take these cells, transfer them in equal numbers into different hosts, and then, provide the antigen, only the IL-7Rahi will be able to survive and proliferate, while the effector cells won't.
Mice infected with LCMV generate a primary CD8 response; some effector cells express high levels of IL-7Ra, while others do not
Only transfer of the IL-7Rghi-CD8 T cells into naive mice led to robust expansion of antigen-specific CD8 cells after secondary challenge
transfer antigen challenge IL-7Rghi cells
LCMV IL-7Rglo cells
.... antigen challenge
0 CD8 0 7 14 Days after transfer naive mice
Student's question: Regarding T cell recognizing self-peptides, would it not lead to autoimmunity?
Professor's answer: This is not a recognition that is enough to trigger their activation. This is a low affinity recognition, yet a recognition: these cells are specific for a pathogen but can cross-react with cell function, but the signal is not sufficient to trigger their effector function, it is just sufficient to allow them to survive.
Student's question: Why do we have recognition of self-peptides? Why is this mechanism required for memory cells to survive?
Professor's answer: Ultimately, timing selection must balance two main things: one is to be able to recognize the widest repertoire of antigens, because we don't know what we are going to encounter, as for example SARS-CoV-2. However, if you push this too much in
Page 4 of 13 Number of antigen-specific CD8 T cells TCR-transgenic mouse transfer