Myelodysplastic Syndrome and Paroxysmal Nocturnal Hemoglobinuria by Prof. Maria Teresa Giaimo
Slides from Prof. Maria Teresa Giaimo about Myelodysplastic Syndrome/neoplasm. The Pdf explores these hematological conditions, including their clinical manifestations, diagnostic criteria, and available therapies. This University-level Biology document provides detailed explanations, supported by diagrams, for a comprehensive understanding of the subject.
See more20 Pages

Unlock the full PDF for free
Sign up to get full access to the document and start transforming it with AI.
Preview
Myelodysplastic Syndrome/Neoplasm Definition
Hematology 22.03.2024 Prof. Maria Teresa Giaimo MYELODYSPLASTIC SYNDROME/NEOPLASM Definition - Myelodysplastic neoplasm, or syndrome, is a peculiar disease of the bone marrow that occurs when haematopoiesis does not take place correctly. Generally speaking, there is an abnormal formation of the myeloid cells in the bone marrow. Myeloid neoplasm is therefore characterized by clonal proliferation of the hematopoietic stem cells with ineffective haematopoiesis - therefore cytopenia (< 10g/dL) in peripheral blood - and recurrent genetic abnormality. It was first described in 1982, and the name of this disease was derived from its characteristics. At the beginning, it was described as something alternative from a morphological point of view. Then, about ten years later, it was discovered that one or more genetic abnormalities were responsible for the formation of a clone of myeloid cells that had abnormal features and couldn't achieve normal maturation. Later on, other genetic findings led to the final definition of the disease: as said before, clonal proliferation of the hematopoietic stem cells with ineffective haematopoiesis and recurrent genetic abnormality.
Pathophysiology of Myelodysplastic Syndrome
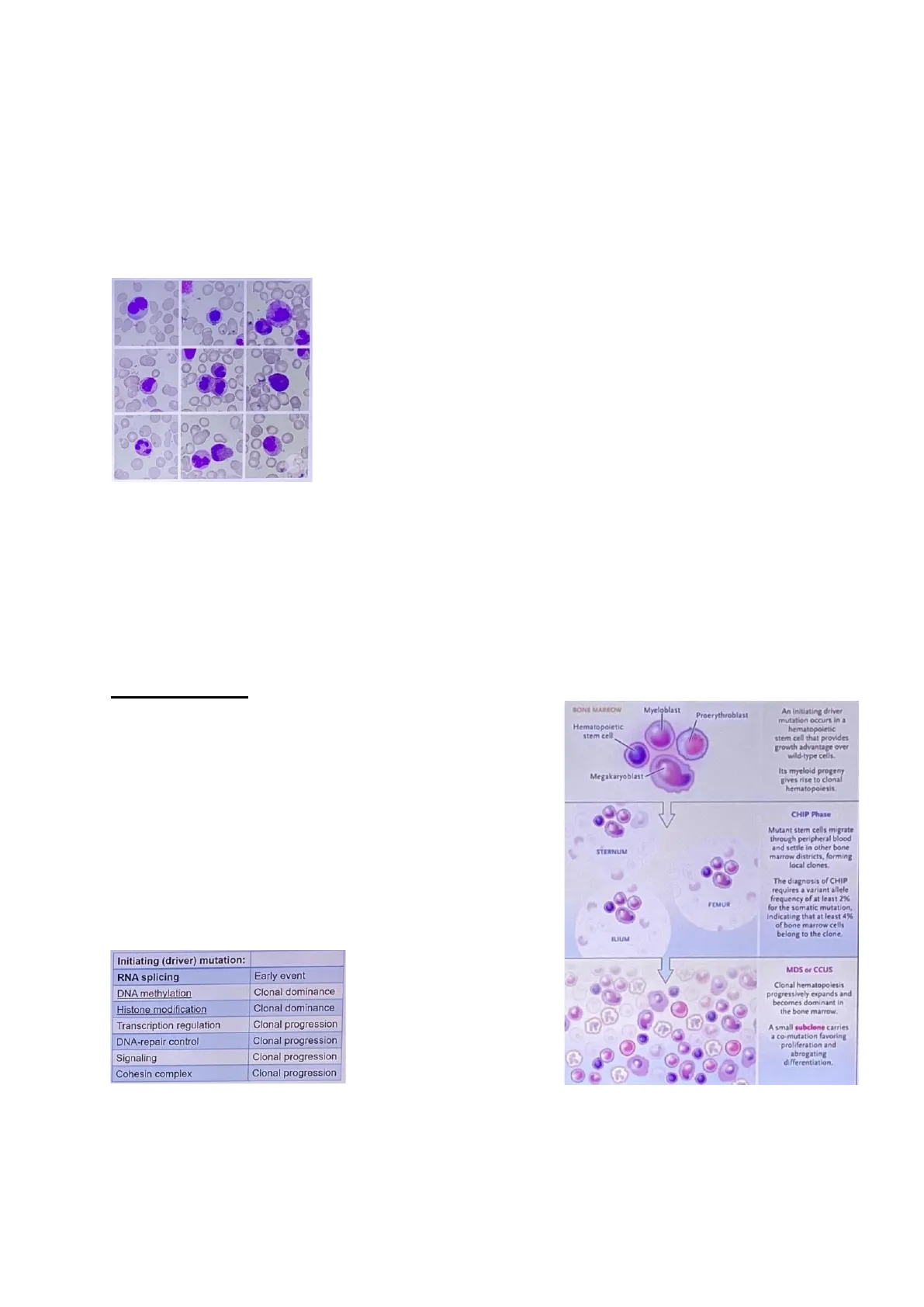
Pathophysiology At the base of the disease, there's a genetic disruption, and hence the factor initiating this disease is a genetic mutation, which most of the time affects genes involved in RNA splicing. Mutation in RNA splicing is defined as an early event, as it is described as the first pathological event within a cell. This first mutation has consequences on other genes, leading to the accumulation of mutations. Other early events are those involving genes related to DNA methylation or histone modification, both causing clonal dominance.
Initiating Driver Mutation and Clonal Progression
Initiating (driver) mutation: MDS or CCUS RNA splicing Early event DNA methylation Clonal dominance Histone modification Clonal dominance Transcription regulation Clonal progression DNA-repair control Clonal progression Signaling Clonal progression Cohesin complex Clonal progression
Bone Marrow Changes in Myelodysplastic Syndrome
BONE MARROW Myeloblast Proerythroblast Hematopoietic stem cell An initiating driver mutation occurs in a hematopoletic stem cell that provides growth advantage over wild-type cells. Megakaryoblast Its myeloid progeny gives rise to clonal hematopoiesis. CHIP Phase STERNUM Mutant stem cells migrate through peripheral blood and settle in other bone marrow districts, forming local clones FEMUR The diagnosis of CHIP requires a variant allele frequency of at least 2% for the somatic mutation, Indicating that at least 4% of bone marrow cells belong to the clone. ILIUM Other mutations are responsible for clonal progression. Clonal hematopoiesis progressively expands and becomes dominant in the bone marrow. A small subclone carries a co-mutation favoring proliferation and abrogating differentiation.
In general, the most frequent genes involved are SF3B1, TET2, SRSF2, AXL1, DNMT3A and RUNX1.Most of the time, the alteration is an age-related event, with a median age of around 70 yo (M>F) and an incidence of 4-5 cases / 100 000 / year. Indeed, it's something that is more common in older age than in youth. Coming back to the early mutations, specifically after the first mutation there is a selection of a clone that can spread to different sites, where also normal cells are. Moreover, as the mutation increases, clones expand exponentially in the bone marrow. Because of this, at the end it is seen a predominance of the mutant clone, coinciding with the point where the neoplasm typically shows up. At this point, there can be either a morphological dysplasia (if one or more of the major myeloid lineages1 are involved) or persistent cytopenia in peripheral blood. To better explain the latter, imagine that if the dysplasia involves red blood cells (or precursors) there may be anaemia or other typical findings related to RBCs. The cutoff set for the cytopenia are:
- Hb < 10 g/dL
- PLT < 100 000 /mmc
- ANC (= granulocytes) < 1800 / mmc
There is a paradox, as there's a clonal predominance in the bone marrow (some blood cells precursors are growing in the bone marrow) whilst in the peripheral blood, it is observed a cytopenia. This is explained by the fact that the clone present in the bone marrow will not allow the normal cell to mature and reach the peripheral blood.
Clinical Presentation of Myelodysplastic Syndrome
Signs and Symptoms
Clinical presentation Signs Symptoms Persistent cytopenia: - Hb < 10 g/dL Fatigue, shortness of breath - And/or PLT < 100 000/mmc Bruising, bleeding - And/or ANC < 1800/mmc Fever, recurrent or persistent infections The main signs are almost all related to persistent cytopenia, and so are the symptoms. Indeed, if for instance a patient is anaemic, he/she will present with fatigue and shortness of breath. Moreover, if a patient is thrombocytopenia, he/she may present with bruising and bleeding while if he/she has problems with the immune system, there might be recurrent or persistent infections. This should be the starting point for diagnosis. 1 By definition, almost 10% of each lineage should be involved to define it as being affected by a morphological dysplasia. However, not all the lineages must be affected in order to diagnose a myelodysplastic syndrome.
Diagnosis of Myelodysplastic Syndrome
Diagnosis Concerning the differential diagnosis, check always that, if a patient shows unexplained blood cytopenia, there are no other causes (e.g., vitamin deficiency or exposure to some specific drugs) that may lead to the same finding. Usually, myelodysplastic syndrome is not always the first diagnosis, as there are several other causes that may induce same (or similar) findings. However, if the patient is old and shows persistent symptoms (> 1 month), the possibility of the disease must be taken into account.
Diagnostic Procedures
Molecular profiling: gene panel assay for somatic mutations, plus gene panel assay for germline mutations in selected patients Unexplained blood cytopenia Morphological assessment of bone marrow and peripheral blood plus cytogenetics - Microscopy Chromosomal abnormalities, bone marrow blasts, haemoglobin level, platelet count and absolute neutrophil count After the recognition of symptoms suggesting a myelodysplastic syndrome, proceed with a morphological assessment of both peripheral blood and bone marrow. The first is very easy to perform and can be either done by a simple physician - not necessarily a haematologist. Apart from the morphological evaluation, other exam should be prescribed, in order to have a clearer clinical picture. Obviously, the final diagnosis is made by evaluating the bone marrow.
Lineage Involvement and Dysplasia
Erythroid lineage -- -- Megakaryocyte lineage --- --- Granulocytic lineage In the slide it can be observed what happens for every lineage that displays dysplastic morphological signs. Recall that the lineages involved are the erythroid lineage, the megakaryocytic lineage and the Granulocytic lineage. The cutoff in order to diagnose a myelodysplastic syndrome is 10%, meaning that whenever it is evaluated the bone marrow, it should be seen at least 10% of each lineage presenting alterations to establish that that lineage is dysplastic. However, keep in mind that it is NOT necessary to have all the lineages involved simultaneously! Indeed, even if just one lineage is involved, a diagnosis of myelodysplastic neoplasm can be made. If the hypothesis is confirmed, proceed with molecular profiling (as this neoplasm is based on genetic alterations, that causes the formation of a clone). Nowadays it is possible to identify which genetic aberration is at the base of the disease, and this information is particularly important for the prognosis of the disease.
Classification of Myelodysplastic Syndromes
Classification In 2022 two new classifications come out, derived from a separation between the WHO classification and the European one. These two are quite similar when consideringmyelodysplastic neoplasms with some differences when dealing with other pathologies, such as in the case of leukaemia.
European Classification of Myelodysplastic Syndromes
European classification The council in charge decided to distinguish several types of myelodysplastic syndromes:
- Low risk, genetically defined myelodysplastic syndrome. Among this class are
found:
- Myelodysplastic syndrome with mutated SF3B1 (a gene involved in RNA splicing). This kind of mutation is - most of the time - the first genetic lesion that appears and it is characterized by a very good prognosis. In the older classification, it was associated with ringed-sideroblasts 2, indeed these are strictly connected to the SF3B1 mutation. Nevertheless, there are about 5%-10% of cases where the ringed-sideroblasts are not accompanied by the mutation.
- Myelodysplastic syndrome with deletion 5q (i.e., chromosome 5 branch q). It is characterized by good prognosis as well; typically is associated with thrombocytosis instead of thrombocytopenia.
- Medium high risk morphological defined myelodysplastic neoplasm.
This group is characterised by the number of lineages involved with, and not by
any particular genetic aberration. Among this class are found:
- Myelodysplastic syndrome with single lineage
- Myelodysplastic syndrome with multiple lineage dysplasia
- Myelodysplastic syndrome with excess blasts3.
- Another entity is myelodysplastic syndrome/ acute myeloid leukaemia - a bridge between the myelodysplastic syndrome and the acute myeloid leukaemia - as it's characterized by 10%- 19% of blasts. This new entity was introduced in order to clarify how to manage a patient. Indeed, a patient diagnosed with this kind of disease can be treated as those that already have acute leukaemia (who already have more than 20% of blasts) as they will eventually develop an acute myeloid leukaemia and preventive therapy might give better outcomes.
- Myelodysplastic syndrome without dysplasia is not characterised by morphological features of the BM common to all the previous types, but instead there is a genetic alteration related to chromosome 7. As a matter of fact, this mutation is the only characteristic that allows to correctly identify that it is a myelodysplastic syndrome - even though the morphological alterations are not yet evident.
- Myelodysplastic syndrome involving the p53 mutation. Recall that P53 is involved in cell cycle regulation, and that when disrupted it can cause very aggressive disease 2 red cell precursor characterized by a ring appearing blue on a histological slide due to the presence of granules with iron inside, typically forming in abnormal hematopoiesis. 3 Usually are called blasts the precursors of the hematopoietic lineage cells that are not normal, as they are the progenitor of the malignant clone, which is related to the evolution toward the acute myeloid leukemia. When there are more than 5% of blasts in the bone marrow or more than 2% in the peripheral blood, this is defined as a myelodysplastic syndrome with excess blasts, going towards an acute myeloid leukemia and related to a higher risk of an aggressive disease.